Sphagnum Restoration on Degraded Blanket and Raised Bogs in the UK Using Micropropagated Source Material: a Review of Progress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE STATUS of GOLDEN PLOVERS in the PEAK PARK, ENGLAND in RELATION to ACCESS and RECREATIONAL DISTURBANCE by D.W.Yalden
3d THE STATUS OF GOLDEN PLOVERS IN THE PEAK PARK, ENGLAND IN RELATION TO ACCESS AND RECREATIONAL DISTURBANCE by D.W.Yalden A survey of all Peak Park moorlands in 1970-75 concerned birds which had moved onto the then located approximately 580-d00 pairs of Golden recent fire site of Totside Moss - I recorded Plovers (P•u•$ •pr•c•r•); on the present only 2 pairs in those 1-km squares, where they county boundaries around half of these are in found 11 pairs. Also the S.E. Cheshire Derbyshire, a few in Cheshire, Staffordshire moorlands have recently been thoroughly studied and Lancashire (16, 6 and 2 pairs, during the preparation of a breeding bird atlas respectively), and the rest in Yorkshire for the county. Where I found 15 pairs in (Yalden 1974). There are very sparse 1970-75, there seem to be only eight pairs now populations of Golden Plovers in S.W. Britain (A. Booth, D.W. Yalden pets. ohs.). and Wales. On Dartmoor, an R.S.P.B. survey found only 14 pairs, and in the national The Pennine Way long distance footpath runs breeding bird survey this species was only along the ridge from Snake Summit south to recorded in 50 (10 km) squares in Wales, (Mudge Mill-Hill and consequently this area has been et a•. 1981, Shatrock 1976): the total Welsh subjected to disturbance from hill-walkers. population was estimated at 600 pairs. Further Here the Golden Plover population has been north in the Pennines, and in Scotland, tensused annually since 1972. The population populations are larger. -

A Year in Review 2019–2020
MOORS FOR THE FUTURE PARTNERSHIP A year in review 2019–2020 Protecting the uplands for the benefit of us all MOORS FOR THE FUTURE PARTNERSHIP Moor business but not as usual It was a busy year for the Partnership, with another record-breaking year of works coming to a close with the wettest February on record, followed by the start of the coronavirus pandemic which led to the suspension of activities a few weeks early. Despite this, the Partnership managed to complete most of our planned conservation works over nearly 2,000 hectares of peatland landscape. Alongside the conservation works, we We gave a presentation at a workshop By David Chapman, assisted the Heather Trust with an event on natural capital organised by Greater Chair of Moors for the for 40 people on Bradfield Moor in the Manchester Combined Authority, as well as Future Partnership Peak District and a follow-up discussion presentations at Care Peat conference, APEM on natural capital. conference on delivering natural capital and We met Environment Agency CEO Sir James at a Manchester Metropolitan University Bevan to demonstrate how much the Agency seminar on how evidence from monitoring has achieved by partnership working. The visit informs our future conservation work. included a trip to Winter Hill, which is to be We attended a reception at the House of restored as part of our Moor Carbon project. Commons on the importance of peatlands, Engagement with local MPs continued with organised by IUCN UK Peatland Programme a visit by Sir Patrick McLoughlin (Derbyshire and Yorkshire Wildlife Trust. Dales). -

Walk the Way in a Day Walk 45 Black Hill from Standedge
Walk the Way in a Day Walk 45 Black Hill from Standedge A challenging walk across open moorland, combining 1965 - 2015 old and new Pennine Way routes. After following an easy path beside reservoirs and up onto Black Hill, the return route crosses dreadful terrain - including the infamous Saddleworth Moor - with difficult navigation making fair weather essential.. Length: 12½ miles (20¼ kilometres) Ascent: 1,657 feet (505 metres) Highest Point: 1,910 feet (582 metres) Map(s): OS Explorer OL Map 1 (‘The Peak District - Dark Peak’) (West Sheet) Starting Point: Standedge parking area, Saddleworth (SE 019 095) Facilities: Inn nearby. Website: http://www.nationaltrail.co.uk/pennine-way/route/walk- way-day-walk-45-black-hill-standedge Wessenden Moor The first part of the walk follows the Pennine Way over Wessenden Moor, a total of 5½ miles (8¾ kilometres). At the parking area, a finger sign points to a path climbing above Standedge Cutting. Joining a track heading east- south-east, this follows the course of an old turnpike, constructed in 1815 and subsequently replaced by the alignment now used by the A62. Off to the left, beneath the shapely form of Pule Hill, is Redbrook Reservoir, built to supply the Huddersfield Narrow Canal. Looking ahead, the Holme Moss transmitter identifies the location of Black Hill. Arriving at an old marker stone, the Pennine Way turns onto a flagged path heading towards a pair of small reservoirs (Black Moss and Swellands) (1 = SE 031 089). Walk 45: Black Hill from Standedge page 1 Holme Moss Transmitter Holmfirth. As the path levels-out, a few cairns confirm the route across the The BBC transmitter at Holme Moss is 750 feet (229 metres) high, plateau. -

Edale, Kinder Scout, Bleaklow and Black Hill: Along the Pennine Way a Weekend Walking Adventure for London-Based Hikers
Edale, Kinder Scout, Bleaklow and Black Hill: along the Pennine Way A weekend walking adventure for London-based hikers 1 of 32 www.londonhiker.com Introduction The Pennine Way: well, what can I say? This is the oldest national trail in the UK, stretching 268 miles from Edale to Kirk Yetholm in Scotland. It is a very famous walk, full of history, atmosphere, adventure, misty wilderness, brooding moorland scenery, and weather-worn rocks! On this weekend you will walk the first two days of the Pennine Way, from Edale to Diggle through the heart of the 'Dark Peak' (so called for its notorious peaty bogs!). This offers a wonderful taster of the trail and takes you into some areas of the countryside familiar Manchester locals over the peak district moorland plateau Kinder Scout, Bleaklow and Black Hill. A third day, continuing along the Pennine Way to Hebden Bridge is described if you wish to extend your trip. This is not for you if like your walking pretty and twee. You certainly don't get pictures of this area on biscuit tins. It's WILD and WINDY and WET and WONDERFUL and GRITTY and GORGEOUS all at once. It's like nowhere else and it'll challenge you in so many ways. This is a very strenusous weekend and the distances are quite long so you need to be confident in your fitness before you do this walk. Ready? Gird your loins! Summary You'll travel up to Edale via either Manchester or Sheffield (see the travel section for more details). -

WEST YORKSHIRE Extracted from the Database of the Milestone Society a Photograph Exists for Milestones Listed Below but Would Benefit from Updating!
WEST YORKSHIRE Extracted from the database of the Milestone Society A photograph exists for milestones listed below but would benefit from updating! National ID Grid Reference Road No. Parish Location Position YW_ADBL01 SE 0600 4933 A6034 ADDINGHAM Silsden Rd, S of Addingham above EP149, just below small single storey barn at bus stop nr entrance to Cringles Park Home YW_ADBL02 SE 0494 4830 A6034 SILSDEN Bolton Rd; N of Silsden Estate YW_ADBL03 SE 0455 4680 A6034 SILSDEN Bolton Rd; Silsden just below 7% steep hill sign YW_ADBL04 SE 0388 4538 A6034 SILSDEN Keighley Rd; S of Silsden on pavement, 100m south of town sign YW_BAIK03 SE 0811 5010 B6160 ADDINGHAM Addingham opp. Bark La in narrow verge, under hedge on brow of hill in wall by Princefield Nurseries opp St Michaels YW_BFHA04 SE 1310 2905 A6036 SHELF Carr House Rd;Buttershaw Church YW_BFHA05 SE 1195 2795 A6036 BRIGHOUSE Halifax Rd, just north of jct with A644 at Stone Chair on pavement at little layby, just before 30 sign YW_BFHA06 SE 1145 2650 A6036 NORTHOWRAM Bradford Rd, Northowram in very high stone wall behind LP39 YW_BFHG01 SE 1708 3434 A658 BRADFORD Otley Rd; nr Peel Park, opp. Cliffe Rd nr bus stop, on bend in Rd YW_BFHG02 SE 1815 3519 A658 BRADFORD Harrogate Rd, nr Silwood Drive on verge opp parade of shops Harrogate Rd; north of Park Rd, nr wall round playing YW_BFHG03 SE 1889 3650 A658 BRADFORD field near bus stop & pedestrian controlled crossing YW_BFHG06 SE 212 403 B6152 RAWDON Harrogate Rd, Rawdon about 200m NE of Stone Trough Inn Victoria Avenue; TI north of tunnel -

Dancing on the Pedals
WHERE The Peak District START & FINISH Stannington, near Sheffield DISTANCE 150km/93 miles WORds Dave Barter PICTURES Dave Barter and Phil O’Connor 1 DANCING ON THE PEDALS The CTC Phil Liggett Challenge Ride on 11 August is packed with classic Peak District climbs – some of which will feature in 2 the 2014 Tour de France. Dave Barter previews the route hil Liggett is the voice of the Tour de France – Peak District has to offer, taking in a number of climbs IN THE PHOTOS among other sporting events. Listening to his 1) Winnats Pass is a monster of that have previously hosted cycling classics, such as P genial banter, it’s hard to imagine that Phil is a climb, with a 20% gradient the Tour of the Peak, as well as the Milk Race. This year capable of dishing out the pain rather than commentating 2) Phil Liggett signs on for an the event is on the 11 August, clashing with my family on it. Yet in his time he was both a racing cyclist and race earlier edition of his ride holiday, so I decided to head out in June for a sneak organiser. As I clawed my way to the top of Winnats Pass, preview. desperately searching the surrounding air for additional molecules of oxygen, I began to understand Phil’s UNDER-PREPARED & OVER-GEARED pedigree. The ride that bears his name is testament to his A few days previously, I’d programmed the longer route eye for a tough event. option into my GPS and taken a casual glance at the The Phil Liggett Challenge Ride used to be known stats. -

No.5, Summer 2017
A Charity registered in England and Wales, no. 1163854 Mille NO. 5 NEWSLETTER SUMMER 2017 Welcome to our fifth Newsletter, keeping all our members in touch with recent events, research, excavation, etc. organised by ourselves and by other groups. This is now a quarterly publication from the RRRA sent initially to members before being made available · Viae on our website. We are happy to consider articles and papers for inclusion in future editions - please contact the Editor. In this edition…….. · ducunt The RRRA and the Roman road at Holtye 2 Mike Haken reports on the current condition of an important site in East Sussex which was excavated and saved for posterity by Ivan Margary. Whispers from the Wolds 4 Alison Spencer tells us a little about a new community archaeology group in the Yorkshire Wolds, their first excavation and future study which RRRA is proud to support. · homines Possible “new” Roman road north of Ebchester near the County 5 Durham/ Northumberland border John Poulter gives a brief outline of the research being carried out on the putative “Proto Dere Street” by Northern Archaeology Group, and invites members to visit the site to see for themselves. Braided Tracks 7 David Staveley takes a look at this phenomenon and how it relates to research into Roman roads using examples from Hampshire and Sussex · per A Roman road through Longdendale 11 Roger Hargreaves describes the discovery of this “new” trans-Pennine Roman road, and presents some of the evidence. · secula Edition No. 6, Autumn 2017 will include Two items scheduled for this edition have been held over until the Autumn. -
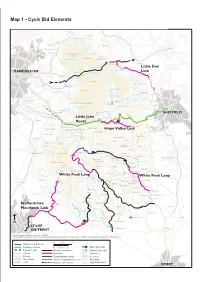
Cycle Bid Elements Map 1 - Cycle Bid Elements
Map 1 - Cycle Bid Elements Map 1 - Cycle Bid Elements A62 Marsden Meltham Butterley Res. Diggle Delph Holmfirth Barnsley Saddleworth A635 Digley Res. Greenfield SADDLEWORTH Holme Oldham MOOR Holme Moss Dove Stone Res. Winscar Res. Dunford Bridge Penistone A6024 ail Langsett Pennine Tr Little Don ans A628 Crowden Tr Langsett Res. MANCHESTER Torside Res. Woodhead Res. Link Trail Stalybridge ale nd Stocksbridge A628 de Tintwistle ng Lo Bottoms Res. Hadfield BLEAKLOW Broomhead Res. Hyde Glossop Howden Res. Snake Pass Charlesworth Bradfield Derwent Res. A624 Strines Res. Stockport A 5 7 Fairholmes Little Hayfield KINDER SCOUT Marple Kinder Res. Ladybower Res. Hayfield Hazel Grove New Mills Edale Stanage Edge SHEFFIELD Little John A6013 Disley Losehill Hall Hope Bamford Poynton Mam Tor Chinley Route Castleton A6187 Lyme Park R. Derwent Whaley Bridge Hathersage Bradwell Kettleshulme Chapel-en-le-Frith Sparrowpit Hope Valley Link Peak Forest Pott Shrigley A623 Grindleford Dronfield A5004Combs Dove Gt. Hucklow Bollington Fernilee Holes Res. Eyam A625 Goyt Foolow Rainow Valley Tideswell Stoney Froggatt Edge Lamaload Middleton Curbar Res. Errwood Litton Res. A537 Monsal Dale Calver A621 Buxton l Tra Macclesfield Macclesfield Cat & Fiddle Monsa il Hassop B6001 Forest Grin Low Lt. Longstone A6 Gt. Longstone Baslow A619 Pilsley Chesterfield Ashford in R. Derwent Taddington A619 A5270 the Water Chelmorton A6020R. Wye. Chatsworth Wildboarclough Edensor Three Shires Flagg A54 Head Sheldon Bakewell Haddon Beeley Flash Hollinsclough A515 B5055 R. Dane Over Rowsley White Peak Loop Monyash Haddon A6 Wincle Lathkill Dale Stanton White Peak Loop The Roaches Longnor in the Peak R. Dove Nine H Darley Dale i Arbor Ladies gh Youlgreave Congleton P Low ea Birchover k Middleton T Robin Hood’s r ail Stride A53 Wensley Hartington Elton Matlock Hulme End Winster Rudyard Lake Tittesworth Res. -

PDP Board Minutes July 2014 FINAL.Docx
Minutes of a Board meeting held on Wednesday 9 July 2014 at 2.00 p.m. at the Agricultural Business Centre, Bakewell Present: Peter Dewhurst (Chair) University of Derby Lesley Stevens High Peak Borough Council Mark Forrester High Peak Borough Council Giles Dann Derbyshire Dales District Council Julie Hirst DCC Public Health Neil Moulden Derbyshire Dales CVS Peter Harrison Peak Park Authority Jonathan Wardle North Derbyshire CCG Ann Wright Derbyshire County Council Jo Battye Derbyshire County Council Graham McLoughlin Derbyshire Police Sylvia Green Rural Action Derbyshire Ian Bates Derbys, Notts & Leics Chamber Alan Dow Tameside & Glossop CCG Rachel Rourke High Peak Borough Council 15/01 WELCOME, APOLOGIES & DECLARATIONS OF INTEREST (Agenda item 1) The Chair welcomed all to this meeting of the PDP, and introductions were made. Apologies for absence were received from: Councillor Bisknell (HPBC), Mark Trillo (HPBC – Mark Forrester is sub), Rachel Gillis (PDNPA), Councillor Dave Wilcox (DCC), Naomi Compton (NDCCG), Dorcas Bunton and Councillor Lewis Rose (DDDC), S Allinson (Tameside & Glossop CCG) 15/02 GOOD NEWS (Agenda item 2) High Peak Borough Council 1. The Council had entered into a concordat with DCC which pledged to work in partnership to drive growth and prosperity 1 2. Glossopdale Trust had been confirmed as a partner for asset transfer to ensure the future of Glossop’s heritage halls, and detailed survey work was now underway 3. Chelsea’s Choice, a play which aimed to raise awareness around child sexual exploitation was being held in Buxton on 10 July 4. Tour de France – reference was made to the successful visit of the Tour de France Derbyshire Dales District Council 1. -

2.1 A62 & A6024 Kirklees
Local Highways Maintenance Challenge Fund Application Form: bids for funding in 2019/20 The level of information provided on this form should be proportionate to the size and complexity of the works proposed. An Excel data proforma should also be completed. Note that DfT funding is a maximum of £5 million per project for bids in 2019-20. An individual local highway authority may apply to bid for only one scheme. Funding will be provided in 2019/20, but it is recognised that construction may go into 2020/21 as well. The closing date for bids is 31 October 2019. For schemes submitted by a Combined Authority for component authorities a separate application form should be completed for each scheme, then the CA should rank them in order of preference. Applicant Information Local authority name: Kirklees Council Bid Manager Name and position: Kathryn Broadbent - Operational Manager Name and position of officer with day to day responsibility for delivering the proposed scheme. Contact telephone number: 07528 988007 Email address: [email protected] Postal address: Highways Service Flint Street Fartown Huddersfield HD1 6LG Postcode Combined Authorities If the bid is from a local highway authority within a Combined Authority, please specify the contact and ensure that the Combined Authority has submitted a Combined Authority Application Ranking Form. Name and position of Combined Authority Bid Co-ordinator: Steve Heckley Contact telephone number: 01132517335 Email address: [email protected] Postal address: West Yorkshire Combined authority, Wellington House, 40-50, Wellington Street, Leeds. LS12DE When authorities submit a bid for funding to the Department, as part of the Government’s commitment to greater openness in the public sector under the Freedom of Information Act 2000 and the Environmental Information Regulations 2004, the local highway authority must also publish a version excluding any commercially sensitive information on their own website within two working days of submitting the final bid to the Department. -

Final Submission Plan, June 2020 2
Holme Valley Neighbourhood Development Plan (NDP) 2020 – 2031 Submission Plan Prepared by the Neighbourhood Plan Steering Group with assistance from Holme Valley NDP Final Submission Plan, June 2020 2 Table of Contents Foreword ............................................................................................... 4 Executive Summary .............................................................................. 6 1.0 Introduction and Background ................................................... 11 2.0 Planning Context for Holme Valley NDP ................................... 16 3.0 Holme Valley NDP Vision and Objectives ................................. 22 4.0 Holme Valley NDP Planning Policies ........................................ 24 4.1 Protecting Local Character ...................................................................... 25 Policy 1: Protecting and Enhancing the Landscape Character of Holme Valley ........... 36 4.2 Conservation Areas and Promoting High Quality Design in New Development ....................................................................................................... 39 Policy 2: Protecting and Enhancing the Built Character of the Holme Valley and Promoting High Quality Design .................................................................................... 61 Holme Valley Parish Actions 1 ..................................................................................... 63 4.3 Conserving and Enhancing Heritage Assets .......................................... 63 Policy 3: Conserving -

Tenements in Lieu of the Several Occupiers Thereof; And
4208 tenements in lieu of the several occupiers thereof; tain place called Smithy Place, in the township and for repealing or for altering, amending, and of Honley, in the parish of Almondbury, in the rendering more effectual some of the powers or west riding of the county of York, and terminating provisions of an Act, passed in the session of Par- near the Gas Works, in the township of Uttoxeter, liament held in the first and second years of the in the parish of Uttoxeter, in the county of Staf- reign of His late Majesty King William the ford, at a point where it is proposed to commence Fourth, intituled " An Act for the better manage- a railway running from Uttoxeter aforesaid to ment of the poor in the several parishes and Dudley, in the county of Worcester, to be called hamlets in the city of Norwich and county of the " the Derbyshire, Staffordshire, and Worcestershire same city," relating to the said rates for the relief Junction Railway," and which said railway or of the poor, and to the assessment, collection, and railways, for which application is intended to be recovery thereof, or of any compositions for the made to Parliament pursuant to this notice, and the same; and which said city, and county of the works and conveniences connected therewith res- same city, comprise the several parishes, hamlets, pectively, will pass or be made from, in, through, liberties, and places following, that is to say; Saint or into the several parishes, townships, townlands, Peter of Mancroft, Saint Peter per Mountergate, and extra-parochial or