Women's Interagency Hiv Study Drug List 1 – By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interbiotech Entecavir
InterBioTech FT-XLS250 Entecavir Product Description Catalog #: XLS250, 5mg XLS251, 10mg XLS252, 50mg XLS253, 100mg AXAIV0, 1ml 10mM in DMSO. Catalog #: Name: Entecavir, Monohydrate Syn: BMS200475 monohydrate; SQ34676 monohydrate CAS : 209216-23-9 MW : 295.29 Formula : C12H17N5O4 Properties : DMSO : ≥ 50 mg/mL (169.33 mM) H2O : 2.8 mg/mL (9.48 mM) >99.5% Storage: Powder: -20°C (long term; possible at +4°C (2 years) (M) Also available: In solvent: -80°C (6 months) -20°C (1 month) Entecavir free form #RO893P/Q/R (Syn.: BMS200475; SQ34676) CAS No. : 142217-69-4; MW: 277.2 For Research Use Only Introduction Entecavir monohydrate (BMS200475 monohydrate; SQ34676 monohydrate) is a potent and selective inhibitor of HBV, with an EC50 of 3.75 nM in HepG2 cell. IC50 & Target EC50:3.75 nM (anti-HBV, HepG2 cell)[1] In Vitro *Solubility : DMSO : ≥ 50 mg/mL (169.33 mM) H2O : 2.8 mg/mL (9.48 mM; Need ultrasonic and warming) *Preparation : 1mM = 1mg in 3.3865 mL Entecavir monohydrate (BMS200475 monohydrate; SQ34676 monohydrate) has a EC50 of 3.75 nM against HBV. It is incorporated into the protein primer of HBV and subsequently inhibits the priming step of the reverse transcriptase. The antiviral activity of BMS-200475 is significantly less against the other RNA and DNA viruses[1]. Entecavir monohydrate is more readily phosphorylated to its active metabolites than other deoxyguanosine analogs (penciclovir, ganciclovir, lobucavir, and aciclovir) or lamivudine. The intracellular half-life of entecavir is 15 h[2]. P.1 InterBioTech FT-XLS250 In Vivo *Preparation : 1. Add each solvent one by one: 10% DMSO 40% PEG300 5% Tween-80 45% saline Solubility: ≥ 3 mg/mL (10.16 mM); Clear solution 2. -

Failure of Initial Antiretroviral Treatment Regimens
An update of Current Research January, 1999 The Forum for Collaborative HIV Research, (FCHR) situated within the Center for Health Policy Research (CHPR) at The George Washington University School of Public Health & Health Services, is an independent public-private partnership composed of representatives from multiple interests in the HIV clinical research arena. The FCHR primarily facilitates ongoing discussion and collaboration between appropriate stakeholders on the development and implementation of new clinical studies in HIV and on the transfer of the results of research into clinical practice. The main purpose of the FCHR is to enhance collaboration between interested groups in order to address the critical unanswered questions regarding the optimal medical management of HIV disease. By encouraging coordination among public and private HIV/AIDS clinical research efforts, the FCHR hopes to integrate these efforts into HIV/AIDS medical care settings. Therefore, studies performed by these various research entities, separately or in cooperation, can begin faster; duplication of efforts can be reduced; patient enrollment and retention can be further facilitated; and costs of getting answers to the critical questions can be shared. At present,the FCHR is staffed by three persons and consists of over one hundred members, representing all facets of the field. These include pharmaceutical companies; public and private third-party payors; health care delivery system groups; government agencies; clinical research centers; and patient advocacy groups. The Director of the FCHR is David Barr. For further information about the Forum for Collaborative HIV Research and its projects, please call William Gist at 202-530-2334 or visit our website at: www.gwumc.edu/chpr and click on HIV Research. -

WO 2018/005909 Al 04 January 2018 (04.01.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/005909 Al 04 January 2018 (04.01.2018) W !P O PCT (51) International Patent Classification: A61P 31/12 (2006 .01) A61K 31/505 (2006 .0 1) A61K 31/4985 (2006.01) (21) International Application Number: PCT/US20 17/040 175 (22) International Filing Date: 30 June 2017 (30.06.2017) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/357,458 0 1 July 2016 (01 .07.2016) US (71) Applicant: VIIV HEALTHCARE COMPANY [US/US]; 25 1 Little Falls Drive, Wilmington, DE 19808 (US). (72) Inventor: SPREEN, William, R.; 5 Moore Drive, Re search Triangle Park, NC 27709-3398 (US). (74) Agent: HAN, William, T. et al; Glaxosmithkline, Glob al Patents, UW2220, 709 Swedeland Road, P.O. Box 1539, King of Prussia, PA 19406-0939 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Revised 4/1/2021 GEORGIA MEDICAID FEE-FOR-SERVICE HIV
GEORGIA MEDICAID FEE-FOR-SERVICE HIV-AIDS PA SUMMARY Preferred (may not be all inclusive) Non-Preferred Abacavir generic Abacavir/lamivudine/zidovudine generic Abacavir/lamivudine generic Aptivus (tipranavir) Complera (emtricitabine/rilpivirine/tenofovir disoproxil Atazanavir capsules generic fumarate) Atripla (efavirenz/emtricitabine/tenofovir disoproxil Crixivan (indinavir) fumarate) Biktarvy (bictegravir/emtricitabine/tenofovir Delstrigo (doravirine/lamivudine/tenofovir disoproxil alafenamide) fumarate) Cimduo (lamivudine/tenofovir disoproxil fumarate) Fuzeon (enfuvirtide) Descovy (emtricitabine/tenofovir alafenamide) Intelence (etravirine) Dovato Invirase (saquinavir) Edurant (rilpivirine)* Lexiva (fosamprenavir) Efavirenz tablets generic Nevirapine extended-release generic Emtriva (emtricitabine) Norvir Powder (ritonavir) Epivir solution (lamivudine) Pifeltro (doravirine) Evotaz (atazanavir/cobicistat)* Reyataz Powder (atazanavir) Genvoya (elvitegravir/cobicistat/emtricitabine/ Ritonavir tablets generic tenofovir alafenamide) Isentress and Isentress HD (raltegravir)* Rukobia (fostemsavir) Juluca (dolutegravir/rilpivirine) Selzentry (maraviroc) Kaletra (lopinavir/ritonavir) Stavudine generic^ Stribild (elvitegravir/cobicistat/emtricitabine/ tenofovir Lamivudine generic disoproxil fumarate) Symfi (efavirenz 600 mg/lamivudine/tenofovir Lamivudine/zidovudine generic disoproxil fumarate) Symfi Lo (efavirenz 400 mg/lamivudine/tenofovir Nevirapine immediate-release tablets generic disoproxil fumarate) Norvir (ritonavir) Temixys (lamivudine/tenofovir -
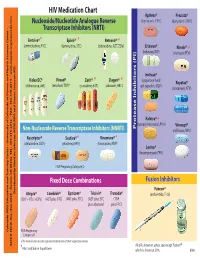
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

HIV-Infected Patients
New Drugs for the Treatment-Experienced Patient Joseph Eron, md Associate Professor of Medicine and Director, Clinical Core unc Center for aids Research, University of North Carolina at Chapel Hill Summary by Tim Horn Edited by Jay Dobkin, md; Michael Saag, md reatment options for antiretro- humans by adenosine deaminase into D- Deeks and his colleagues in 1998 demon- viral-experienced patients leave a dioxolane guanine (dxg), a metabolite that strated a 1 log reduction in hiv-rna in hiv- lot to be desired. According to Dr. has potent activity against hiv and hbv. infected patients—more than 50% of whom Joe Eron, patients who have treat- According to in vitro data presented at were treatment-experienced—who received ment experience in all three classes the 3rd International Workshop on hiv tenofovir df 300 mg once daily as of currently available antiretrovi- Drug Resistance and Treatment Strategies, monotherapy for 28 days (Deeks, 1998). Trals have, at best, a 30% chance of re- held in June 1999, dapd was found to in- According to in vitro data presented by ducing their viral load to levels below 400 hibit wild-type and mutant isolates resistant Gilead’s Dr. Michael Miller at the recent copies/mL upon initiating a salvage regi- to azt (Retrovir) and 3TC (Borroto-Esoda, 4th International Resistance Workshop, the men. Cross-resistance within each class of 1999). The drug was also reported to be ac- resistance pattern for tenofovir df is simi- drugs, particularly the protease inhibitors tive against strains collected from patients lar to that of its chemical predecessor adefo- (pis) and non-nucleoside reverse tran- who have failed various nrti and nnrti vir, a compound no longer in development scriptase inhibitors (nnrtis), essentially combination therapies, including those for the treatment of hiv (Miller, 2000). -

Download Article PDF/Slides
Kan Lu, PharmD New Antiretrovirals for Based on a presentation at prn by Roy M. Gulick, md, mph the Treatment of HIV: Kan Lu, PharmD | Drug Development Fellow University of North Carolina School of Pharmacy Chapel Hill, North Carolina The View in 2006 Roy M. Gulick, md, mph Reprinted from The prn Notebook® | october 2006 | Dr. James F. Braun, Editor-in-Chief Director, Cornell Clinical Trials Unit | Associate Professor of Medicine, Meri D. Pozo, PhD, Managing Editor. Published in New York City by the Physicians’ Research Network, Inc.® Weill Medical College of Cornell University | New York, New York John Graham Brown, Executive Director. For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©october 2006 substantial progress continues to be made in the arena of cokinetics and a long extracellular half-life of approximately 10 hours antiretroviral drug development. prn is again proud to present its annual (Zhu, 2003). During apricitabine’s development, a serious drug interac- review of the experimental agents to watch for in the coming months and tion with lamivudine (Epivir) was noted. Although the plasma years. This year’s review is based on a lecture by Dr. Roy M. Gulick, a long- concentrations of apricitabine were unaffected by coadministration of time friend of prn, and no stranger to the antiretroviral development lamivudine, the intracellular concentrations of apricitabine were reduced pipeline. by approximately sixfold. Additionally, the 50% inhibitory concentration To date, twenty-two antiretrovirals have been approved by the Food (ic50) of apricitabine against hiv with the M184V mutation was increased and Drug Administration (fda) for the treatment of hiv infection. -

Cenicriviroc CSF Abstract Page 1 of 3 Title: Cerebrospinal Fluid Exposure
Cenicriviroc CSF Abstract Title: Cerebrospinal fluid exposure of cenicriviroc in HIV-positive individuals with cognitive impairment Authors: Jasmini Alagaratnam1, Laura Else2, Sujan Dilly Penchala2, Elizabeth Challenger2, Ken Legg1, Claire Petersen1, Brynmor Jones3, Ranjababu Kulasegaram4, Star Seyedkazemi5, Eric Lefebvre5, Saye Khoo2 and Alan Winston1 1. Division of Infectious Diseases, Department of Medicine, Imperial College London, London W2 1PG 2. Department of Pharmacology, University of Liverpool, Liverpool L69 7SX, UK 3. Department of Radiology, Imperial College Healthcare NHS Trust, London W2 1NY 4. St. Thomas’ Hospital, London W6 8RP, UK 5. Clinical Development, Allergan plc, South San Francisco, USA Page 1 of 3 Cenicriviroc CSF Abstract Abstract Background: Cenicriviroc, a dual C-C chemokine receptor type 2 (CCR2) and type 5 (CCR5) antagonist, is a potential adjunctive therapy, along with antiretroviral therapy (ART), for the management of HIV-associated cognitive disorders. Materials and Methods: Virologically suppressed persons living with HIV (PLWH) with a clinical diagnosis of HIV-related cognitive impairment intensified ART with cenicriviroc once daily, dose dependent on current ART, for eight weeks. Subjects with current or previous use of CCR5 inhibitors were not eligible. We assessed cerebrospinal fluid (CSF) exposure of cenicriviroc and CSF albumin at week 8, and changes in cognitive function over 8 weeks. Cenicriviroc concentration was determined using reverse phase high-performance liquid chromatography (HPLC) with geometric mean (GM) and 95% confidence intervals (CI) calculated. The proposed cenicriviroc target concentration was above the 90% effective concentration (EC90) for cenicriviroc (0.17 ng/mL), with the lower limit of quantification (LLQ) 0.24 ng/mL taken as target concentration. -

Chemical Properties Biological Description Solubility Information
Data Sheet (Cat.No.T0085L) Entecavir Chemical Properties CAS No.: 142217-69-4 Formula: C12H15N5O3 Molecular Weight: 277.28 Appearance: Solid Storage: 0-4℃ for short term (days to weeks), or -20℃ for long term (months). Biological Description Description Entecavir is a guanosine nucleoside analogue used in the treatment of chronic hepatitis B virus (HBV) infection. Entecavir therapy can be associated with flares of the underlying hepatitis B during or after therapy, but has not been linked to cases of clinically apparent liver injury. Targets(IC50) HBV( HepG2 cell): EC50:3.75nM In vitro BMS-200475 has a EC50 of 3.75 nM against HBV. It is incorporated into the protein primer of HBV and subsequently inhibits the priming step of the reverse transcriptase. The antiviral activity of BMS-200475 is significantly less against the other RNA and DNA viruses[1]. Entecavir is more readily phosphorylated to its active metabolites than other deoxyguanosine analogs (penciclovir, ganciclovir, lobucavir, and aciclovir) or lamivudine. The intracellular half-life of entecavir is 15 h[2]. In vivo Daily oral treatment with BMS-200475 at doses ranging from 0.02 to 0.5 mg/kg of body weight for 1 to 3 months effectively reduces the level of woodchuck hepatitis virus (WHV) viremia in chronically infected woodchucks[3]. Cell Research BMS 200475 is prepared in phosphate-buffered saline (PBS) and diluted with appropriate medium containing 2% fetal bovine serum. HepG2 2.2.15 cells are plated at a density of 5×105 cells per well on 12-well Biocoat collagen-coated plates and are maintained in a confluent state for 2 to 3 days before being overlaid with 1 mL of medium spiked with BMS 200475. -

( 12 ) United States Patent
US010385067B2 (12 ) United States Patent ( 10 ) Patent No. : US 10 , 385, 067 B2 Carra et al. (45 ) Date of Patent: Aug. 20 , 2019 (54 ) SODIUM (2R , 55 , 13AR ) - 7 , 9 -DIOX0 - 10 ( 56 ) References Cited ( 2 , 4 ,6 - TRIFLUOROBENZYL )CARBAMOYL ) 2 , 3 , 4 , 5 , 7 , 9 , 13 , 13A -OCTAHYDRO - 2 , 5 U . S . PATENT DOCUMENTS METHANOPYRIDO [ 1 ' , 2 ' : 4 , 5 ]PYRAZINO 5 , 814 ,639 A 9 / 1998 Liotta et al . [ 2 , 1 - B ] [ 1 , 3 ]OXAZEPIN - 8 - OLATE 5 , 914 , 331 A 6 / 1999 Liotta et al . 5 ,922 ,695 A 7 / 1999 Arimilli et al . 5 , 935 , 946 A 8 / 1999 Munger, Jr . et al. (71 ) Applicant: Gilead Sciences , Inc ., Foster Ctiy , CA 5 , 977 , 089 A 11/ 1999 Arimilli et al. (US ) 6 ,043 , 230 A 3 / 2000 Arimilli et al. 6 ,620 , 841 B1 9 / 2003 Fujishita et al . (72 ) Inventors : Ernest A . Carra , Foster City , CA ( US ) ; 6 ,642 , 245 B1 11/ 2003 Liotta et al. 6 , 703 , 396 B1 3 / 2004 Liotta et al . Irene Chen , San Mateo , CA (US ) ; 7 , 176 , 220 B2 2 /2007 Satoh et al. Vahid Zia , Palo Alto , CA (US ) 7 ,419 , 969 B2 9 / 2008 Naidu et al. 7 , 550 , 463 B2 6 / 2009 Yoshida (73 ) Assignee : Gilead Sciences , Inc. , Foster City , CA 7 ,635 , 704 B2 12 /2009 Satoh et al. 7 , 858 , 788 B2 12 / 2010 Yoshida et al . (US ) 8 , 129 , 385 B2 3 / 2012 Johns et al . 8 , 148 , 374 B2 4 / 2012 Desai et al. ( * ) Notice : Subject to any disclaimer , the term of this 8 , 188 , 271 B2 5 / 2012 Yoshida et al . -

Ep 2531027 B1
(19) TZZ ¥_Z _T (11) EP 2 531 027 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/4985 (2006.01) A61K 31/52 (2006.01) 06.05.2015 Bulletin 2015/19 A61K 31/536 (2006.01) A61K 31/513 (2006.01) A61K 38/55 (2006.01) A61P 31/18 (2006.01) (21) Application number: 11737484.3 (86) International application number: (22) Date of filing: 24.01.2011 PCT/US2011/022219 (87) International publication number: WO 2011/094150 (04.08.2011 Gazette 2011/31) (54) Therapeutic combination comprising dolutegravir, abacavir and lamivudine Therapeutische Zusammensetzung enthaltend Dolutegravir, Abacavir und Lamivudine Combinaison thérapeutique comprenant du dolutégravir, de l’abacavir et de la lamivudine (84) Designated Contracting States: (74) Representative: Gladwin, Amanda Rachel AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GlaxoSmithKline GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Global Patents (CN925.1) PL PT RO RS SE SI SK SM TR 980 Great West Road Designated Extension States: Brentford, Middlesex TW8 9GS (GB) BA ME (56) References cited: (30) Priority: 27.01.2010 US 298589 P WO-A1-2010/011812 WO-A2-2009/148600 US-A1- 2006 084 627 US-A1- 2006 084 627 (43) Date of publication of application: US-A1- 2008 076 738 US-A1- 2009 318 421 12.12.2012 Bulletin 2012/50 US-A1- 2009 318 421 US-B1- 6 544 961 (73) Proprietor: VIIV Healthcare Company • SONG1 et al: "The Effect of Ritonavir-Boosted Research Triangle Park, NC 27709 (US) ProteaseInhibitors on the HIV Integrase Inhibitor, S/GSK1349572,in Healthy Subjects", INTERNET , (72) Inventor: UNDERWOOD, Mark, Richard 15 September 2009 (2009-09-15), XP002697436, Research Triangle Park Retrieved from the Internet: URL:http: North Carolina 27709 (US) //www.natap.org/2009/ICCAC/ICCAC_ 52.htm [retrieved on 2013-05-21] Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Novel Therapeutics for Epstein–Barr Virus
molecules Review Novel Therapeutics for Epstein–Barr Virus Graciela Andrei *, Erika Trompet and Robert Snoeck Laboratory of Virology and Chemotherapy, Department of Microbiology and Immunology, Rega Institute for Medical Research, KU Leuven, 3000 Leuven, Belgium; [email protected] (E.T.); [email protected] (R.S.) * Correspondence: [email protected]; Tel.: +32-16-321-915 Academic Editor: Stefano Aquaro Received: 15 February 2019; Accepted: 4 March 2019; Published: 12 March 2019 Abstract: Epstein–Barr virus (EBV) is a human γ-herpesvirus that infects up to 95% of the adult population. Primary EBV infection usually occurs during childhood and is generally asymptomatic, though the virus can cause infectious mononucleosis in 35–50% of the cases when infection occurs later in life. EBV infects mainly B-cells and epithelial cells, establishing latency in resting memory B-cells and possibly also in epithelial cells. EBV is recognized as an oncogenic virus but in immunocompetent hosts, EBV reactivation is controlled by the immune response preventing transformation in vivo. Under immunosuppression, regardless of the cause, the immune system can lose control of EBV replication, which may result in the appearance of neoplasms. The primary malignancies related to EBV are B-cell lymphomas and nasopharyngeal carcinoma, which reflects the primary cell targets of viral infection in vivo. Although a number of antivirals were proven to inhibit EBV replication in vitro, they had limited success in the clinic and to date no antiviral drug has been approved for the treatment of EBV infections. We review here the antiviral drugs that have been evaluated in the clinic to treat EBV infections and discuss novel molecules with anti-EBV activity under investigation as well as new strategies to treat EBV-related diseases.