Download Leaflet View the Patient Leaflet in PDF Format
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Antibiotic Resistance in Plant-Pathogenic Bacteria
PY56CH08-Sundin ARI 23 May 2018 12:16 Annual Review of Phytopathology Antibiotic Resistance in Plant-Pathogenic Bacteria George W. Sundin1 and Nian Wang2 1Department of Plant, Soil, and Microbial Sciences, Michigan State University, East Lansing, Michigan 48824, USA; email: [email protected] 2Citrus Research and Education Center, Department of Microbiology and Cell Science, Institute of Food and Agricultural Sciences, University of Florida, Lake Alfred, Florida 33850, USA Annu. Rev. Phytopathol. 2018. 56:8.1–8.20 Keywords The Annual Review of Phytopathology is online at kasugamycin, oxytetracycline, streptomycin, resistome phyto.annualreviews.org https://doi.org/10.1146/annurev-phyto-080417- Abstract 045946 Antibiotics have been used for the management of relatively few bacterial Copyright c 2018 by Annual Reviews. plant diseases and are largely restricted to high-value fruit crops because of Access provided by INSEAD on 06/01/18. For personal use only. All rights reserved the expense involved. Antibiotic resistance in plant-pathogenic bacteria has Annu. Rev. Phytopathol. 2018.56. Downloaded from www.annualreviews.org become a problem in pathosystems where these antibiotics have been used for many years. Where the genetic basis for resistance has been examined, antibiotic resistance in plant pathogens has most often evolved through the acquisition of a resistance determinant via horizontal gene transfer. For ex- ample, the strAB streptomycin-resistance genes occur in Erwinia amylovora, Pseudomonas syringae,andXanthomonas campestris, and these genes have pre- sumably been acquired from nonpathogenic epiphytic bacteria colocated on plant hosts under antibiotic selection. We currently lack knowledge of the effect of the microbiome of commensal organisms on the potential of plant pathogens to evolve antibiotic resistance. -

Blepharitis Last Revised in October 2015
Menu Sign in (https://accounts.nice.org.uk/signin) Search... Blepharitis Last revised in October 2015 Changes Back to top Last revised in October 2015 October 2015 — reviewed. A literature search was conducted in October 2015 to identify evidence based guidelines, UK policy, systematic reviews, and key randomized controlled trials published since the last revision of the topic. No changes to clinical recommendations have been made. Previous changes Back to top September 2012 — reviewed. A literature search was conducted in September 2012 to identify evidencebased guidelines, UK policy, systematic reviews, and key RCTs published since the last revision of the topic. No changes to clinical recommendations have been made. April 2011 — minor update. Change to recommendation regarding need for additional contraception during or after a course of tetracycline additional contraception is no longer required when using antibiotics that are not enzyme inducers with combined hormonal methods for durations of 3 weeks or less [FSRH, 2011 (/blepharitis#!references/315093)]. Issued in June 2011. March 2011 — topic structure revised to ensure consistency across CKS topics — no changes to clinical recommendations have been made. September 2010 — minor update. Hydromoor® (hypromellose 0.3% preservativefree single dose eye drops) have been included. Issued in September 2010. March 2010 — minor update. LubriTears® eye ointment has been discontinued. Prescription removed. Issued in March 2010. December 2007 to May 2008 — converted from CKS guidance to CKS topic structure. The evidence base has been reviewed in detail, and recommendations are more clearly justified and transparently linked to the supporting evidence. The clinical scenario structure has been reviewed. -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Rimicorim 300mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 408 mg of lymecycline equivalent to 300mg tetracycline’ For a full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Capsules, Hard Hard gelatin capsule with yellow body and red cap imprinted with “NM”. 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications - Lymecycline is indicated for the treatment of moderate to severe acne vulgaris (please see section 4.4 and 5.1). Consideration should be given to official guidance on the appropriate use of antibacterial agents. 4.2 Posology and method of administration Moderate to severe acne vulgaris: Adults: The usual dosage for the long-term treatment of moderate to severe acne is 1 capsule daily taken with at least half a glass of water whilst in an upright position. It should be taken with a light meal without dairy products. Treatment should be continued for at least 8 weeks to 12 weeks, however it is important to limit the use of antibiotics to the shortest possible period and discontinue their use when further improvement is unlikely. The treatment should not exceed a duration of 6 months. Elderly: As for other tetracyclines, no specific dose adjustment is required. Paediatric population: The safety and efficacy of Lymecycline in children aged under 12 years of age have not been established. No data are available. For children over the age of 12 years the adult dosage may be given. For the children under the age of 8 years, see section 4.3. -

E3 Appendix 1 (Part 1 of 2): Search Strategy Used in MEDLINE
This single copy is for your personal, non-commercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, contact CJHP at [email protected] Appendix 1 (part 1 of 2): Search strategy used in MEDLINE # Searches 1 exp *anti-bacterial agents/ or (antimicrobial* or antibacterial* or antibiotic* or antiinfective* or anti-microbial* or anti-bacterial* or anti-biotic* or anti- infective* or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or clindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).kf,kw,ti. or (antimicrobial or antibacterial or antiinfective or anti-microbial or anti-bacterial or anti-infective or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or c lindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).ab. /freq=2 2 alamethicin/ or amdinocillin/ or amdinocillin pivoxil/ or amikacin/ or amoxicillin/ or amphotericin b/ or ampicillin/ or anisomycin/ or antimycin a/ or aurodox/ or azithromycin/ or azlocillin/ or aztreonam/ or bacitracin/ or bacteriocins/ or bambermycins/ or bongkrekic acid/ or brefeldin a/ or butirosin sulfate/ or calcimycin/ or candicidin/ or capreomycin/ or carbenicillin/ or carfecillin/ or cefaclor/ or cefadroxil/ or cefamandole/ or cefatrizine/ or cefazolin/ or cefixime/ or cefmenoxime/ or cefmetazole/ or cefonicid/ or cefoperazone/ -

Danmap 2006.Pmd
DANMAP 2006 DANMAP 2006 DANMAP 2006 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark Statens Serum Institut Danish Veterinary and Food Administration Danish Medicines Agency National Veterinary Institute, Technical University of Denmark National Food Institute, Technical University of Denmark Editors: Hanne-Dorthe Emborg Danish Zoonosis Centre National Food Institute, Technical University of Denmark Mørkhøj Bygade 19 Contents DK - 2860 Søborg Anette M. Hammerum National Center for Antimicrobials and Contributors to the 2006 Infection Control DANMAP Report 4 Statens Serum Institut Artillerivej 5 DK - 2300 Copenhagen Introduction 6 DANMAP board: National Food Institute, Acknowledgements 6 Technical University of Denmark: Ole E. Heuer Frank Aarestrup List of abbreviations 7 National Veterinary Institute, Tecnical University of Denmark: Sammendrag 9 Flemming Bager Danish Veterinary and Food Administration: Summary 12 Justin C. Ajufo Annette Cleveland Nielsen Statens Serum Institut: Demographic data 15 Dominique L. Monnet Niels Frimodt-Møller Anette M. Hammerum Antimicrobial consumption 17 Danish Medicines Agency: Consumption in animals 17 Jan Poulsen Consumption in humans 24 Layout: Susanne Carlsson Danish Zoonosis Centre Resistance in zoonotic bacteria 33 Printing: Schultz Grafisk A/S DANMAP 2006 - September 2007 Salmonella 33 ISSN 1600-2032 Campylobacter 43 Text and tables may be cited and reprinted only with reference to this report. Resistance in indicator bacteria 47 Reprints can be ordered from: Enterococci 47 National Food Institute Escherichia coli 58 Danish Zoonosis Centre Tecnical University of Denmark Mørkhøj Bygade 19 DK - 2860 Søborg Resistance in bacteria from Phone: +45 7234 - 7084 diagnostic submissions 65 Fax: +45 7234 - 7028 E. -

In Silico and in Vitro Screening of FDA-Approved Drugs for Potential Repurposing Against Tuberculosis
bioRxiv preprint doi: https://doi.org/10.1101/228171; this version posted December 3, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. In silico and in vitro screening of FDA-approved drugs for potential repurposing against tuberculosis Sridharan Brindha1, Jagadish Chandrabose Sundaramurthi2, Savariar Vincent1, Devadasan Velmurugan3, John Joel Gnanadoss1* 1Loyola College, Nungambakkam, Chennai 600034, Tamil Nadu, India 2National Institute for Research in Tuberculosis (ICMR), Chetpet, Chennai 600031, Tamil Nadu, India 3CAS in Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai – 600025, Tamil Nadu, India *Corresponding Author: Dr. John Joel Gnanadoss Assistant Professor Department of Biotechnology Loyola College (University of Madras) Nungambakkam, Chennai – 600034 Tamil Nadu, India. Phone: 9840985870 E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/228171; this version posted December 3, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Motivation Repurposing of known drugs to newer clinical conditions is a promising avenue for finding novel therapeutic applications for tuberculosis. Methods We performed docking-based virtual screening for 1554 known drugs against two of the potential drug targets, namely trpD and coaA of M. tuberculosis. In the first round of in silico screening we used rigid docking using Glide and AutoDock Vina. We subjected the consistently ranked drugs for induced-fit docking by these tools against the same target proteins. We performed luciferase reporter phage (LRP) assay to determine the biological activity of five selected drugs against M. -

Surveillance of Antimicrobial Consumption in Europe 2013-2014 SURVEILLANCE REPORT
SURVEILLANCE REPORT SURVEILLANCE REPORT Surveillance of antimicrobial consumption in Europe in Europe consumption of antimicrobial Surveillance Surveillance of antimicrobial consumption in Europe 2013-2014 2012 www.ecdc.europa.eu ECDC SURVEILLANCE REPORT Surveillance of antimicrobial consumption in Europe 2013–2014 This report of the European Centre for Disease Prevention and Control (ECDC) was coordinated by Klaus Weist. Contributing authors Klaus Weist, Arno Muller, Ana Hoxha, Vera Vlahović-Palčevski, Christelle Elias, Dominique Monnet and Ole Heuer. Data analysis: Klaus Weist, Arno Muller and Ana Hoxha. Acknowledgements The authors would like to thank the ESAC-Net Disease Network Coordination Committee members (Marcel Bruch, Philippe Cavalié, Herman Goossens, Jenny Hellman, Susan Hopkins, Stephanie Natsch, Anna Olczak-Pienkowska, Ajay Oza, Arjana Tambić Andrasevic, Peter Zarb) and observers (Jane Robertson, Arno Muller, Mike Sharland, Theo Verheij) for providing valuable comments and scientific advice during the production of the report. All ESAC-Net participants and National Coordinators are acknowledged for providing data and valuable comments on this report. The authors also acknowledge Gaetan Guyodo, Catalin Albu and Anna Renau-Rosell for managing the data and providing technical support to the participating countries. Suggested citation: European Centre for Disease Prevention and Control. Surveillance of antimicrobial consumption in Europe, 2013‒2014. Stockholm: ECDC; 2018. Stockholm, May 2018 ISBN 978-92-9498-187-5 ISSN 2315-0955 -

246. Minocycline 2.0.Pdf
246 | February 2020 | 2.0 Community Interest Company Minocycline prescribing Nationally £15.1 million is spent annually on tetracyclines for acne, of which approximately £457,000 is spent on minocycline (ePACT July to September 2019). QIPP projects in this area are aimed at reviewing the continued need for oral antibiotics and switching to an alternative with better safety and efficacy and a lower acquisition cost. This bulletin reviews the place in therapy of minocycline and offers guidance and support material for organisations considering reviewing their prescribing as a QIPP project. Recommendations • Do not commence new patients requiring oral antibiotic acne treatment on minocycline. • Commence new patients requiring oral acne treatment on a once daily tetracycline antibiotic with a better safety profile than minocycline (doxycycline or lymecycline). Once daily treatments may provide better adherence. • Review all patients on oral antibiotic treatment for acne for continued need after 3 months. • Do not prescribe oral acne treatment for patients with mild acne. • Review all patients on minocycline for continued need and discontinue treatment if no longer indicated. • For patients on minocycline where treatment for acne is still indicated, review for suitability of switching to an alternative treatment. Switch all suitable patients to an alternative treatment. As with all switches, these should be tailored to the individual patient. National guidance NICE published a Key Therapeutic Topic bulletin entitled Minocycline in January -

Skin/Soft Tissue Infections Illness Comments Drug Dose Duration Of
Skin/Soft Tissue Infections Illness Comments Drug Dose Duration of Tx Note: doses are oral and for adults unless otherwise stated. Please refer to BNF for further information. Impetigo Topical use should be minimised to Flucloxacillin 500mg QDS 7 days reduce development of resistance. or Clarithromycin 250mg-500mg BD 7 days Caution: with the topical use of fusidic acid as there may be local resistance. For minor infections only: Multiple courses of topical antibiotics Hydrogen peroxide cream 1% 2-3 times daily Up to 3 weeks may lead to increased resistance. Second line: Fusidic acid Topically TDS 5 days Cellulitis Class 1: patients have no signs or symptoms of systemic toxicity and have no uncontrolled co- morbidities and are managed on an outpatient basis with oral antibiotics. Class 2: patients are either systemically ill, without any unstable co-morbidities, or are systemically well, but have one or more co-morbidities. Require initial parenteral antibiotic therapy which may be delivered from home if OPAT services available Class 3 and 4: patients are septic or, have at least one unstable co-morbidity, or a limb- threatening infection. Require admission to hospital for parenteral antibiotic therapy. Return to Top Skin/Soft Tissue Infections Illness Comments Drug Dose Duration of Tx Note: doses are oral and for adults unless otherwise stated. Please refer to BNF for further information. Manage underlying pre-disposing conditions if any (e.g. tinea pedis, ulcers, lymphoedema). Consider urgent hospital admission for intravenous antibiotic treatment in severe, or rapidly worsening infection; suspected orbital, or periorbital cellulitis; facial cellulitis in a child - maintain a low threshold for hospital admission; immunocompromised; diabetes mellitus - admission may not be necessary if diabetes is stable, but maintain a low threshold for hospital admission; significant co-morbidity (e.g. -

NORM Normgvet
2O16 NORM NORM-VET Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway DESIGN, FORSIDE: IDA SKAAR IDA FORSIDE: DESIGN, – SVANEGODKJENT TRYKKSAK – 241 762 241 – TRYKKSAK SVANEGODKJENT – ISSN: 1502-2307 (print) / 1890-9965 (electronic) AS MEDIA LUNDBLAD 2016 NORM NORM-VET Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway ISSN: 1502-2307 (print) / 1890-9965 (electronic) Any use of data from NORM/NORM-VET 2016 should include specific reference to this report. Suggested citation: NORM/NORM-VET 2016. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø / Oslo 2017. ISSN:1502-2307 (print) / 1890-9965 (electronic). This report is available at www.vetinst.no and www.antibiotikaresistens.no 1 CONTENTS NORM / NORM-VET 2016 CONTRIBUTORS AND PARTICIPANTS Editors: Gunnar Skov Simonsen NORM, Univ. Hosp. North Norway Anne Margrete Urdahl NORM-VET, Norwegian Veterinary Institute Authors: Per Espen Akselsen Antibiotic usage in humans [email protected] KAS, Haukeland Univ. Hosp. Cecilie Torp Andersen Candida spp. [email protected] Oslo Univ. Hosp. Elisabeth Astrup MRSA in humans [email protected] Norw. Inst. of Pub. Health Hege Salvesen Blix Antibiotic usage in humans [email protected] Norw. Inst. of Pub. Health Dominique Caugant Meningococci [email protected] Norw. Inst. of Pub. Health Petter Elstrøm MRSA in humans [email protected] Norw. Inst. of Pub. Health Hege Enger MRSA in humans [email protected] St. Olav Univ. Hosp. Frode Width Gran MRSA in humans [email protected] St. Olav Univ. Hosp. Kari Grave Antibiotic usage in animals [email protected] Norw. -
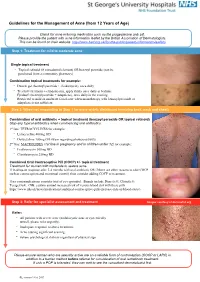
Guidelines for the Management of Acne (From 12 Years of Age)
Guidelines for the Management of Acne (from 12 Years of Age) Check for acne inducing medication such as the progesterone only pill. Please provide the patient with acne information leaflet by the British Association of Dermatologists. This can be found on their website: http://www.bad.org.uk/for-the-public/patient-information-leaflets Step 1: Treatment for mild to moderate acne Single topical treatment - Topical retinoid (if comedomal element) OR benzoyl peroxide (can be purchased from a community pharmacy) Combination topical treatments for example: - Duac® gel (benzoyl peroxide + clindamycin), once daily - Treclin® (tretinoin + clindamycin), apply thinly once daily at bedtime Epiduo® (benzoylperoxide + adapalene), once daily in the evening. Restricted to mild or moderate facial acne when monotherapy with benzoyl peroxide or adapalene is not sufficient. StepStep 2:2: WhenWhen notnot respondingresponding toto StepStep 11 (orand more 2 (or widely more widelydistributed distributed involving involving back, neckback, and neck chest) and chest) Combination of oral antibiotic + topical treatment (benzoyl peroxide OR topical retinoid) Stop any topical antibiotics when commencing oral antibiotics 1st line: TETRACYCLINES for example: - Lymecycline 408mg OD - Doxycycline 100mg OD (warn regarding photosensitivity) 2nd line: MACROLIDES (1st line in pregnancy and in children under 12) for example: - Erythromycin 500mg BD - Clarithromycin 250mg BD Combined Oral Contraceptive Pill (COCP) +/- topical treatment Treatment for women with moderate to severe acne. If inadequate response after 3-4 months with oral antibiotic OR if there are other reasons to take COCP such as contraception and menstrual control, then consider adding COCP to treatment. If no contraindications consider trial of co-cyprindiol. Brands include Dianette®, Clairette®, Teragezza®. -

Summary Report on Antimicrobials Dispensed in Public Hospitals
Summary Report on Antimicrobials Dispensed in Public Hospitals Year 2014 - 2016 Infection Control Branch Centre for Health Protection Department of Health October 2019 (Version as at 08 October 2019) Summary Report on Antimicrobial Dispensed CONTENTS in Public Hospitals (2014 - 2016) Contents Executive Summary i 1 Introduction 1 2 Background 1 2.1 Healthcare system of Hong Kong ......................... 2 3 Data Sources and Methodology 2 3.1 Data sources .................................... 2 3.2 Methodology ................................... 3 3.3 Antimicrobial names ............................... 4 4 Results 5 4.1 Overall annual dispensed quantities and percentage changes in all HA services . 5 4.1.1 Five most dispensed antimicrobial groups in all HA services . 5 4.1.2 Ten most dispensed antimicrobials in all HA services . 6 4.2 Overall annual dispensed quantities and percentage changes in HA non-inpatient service ....................................... 8 4.2.1 Five most dispensed antimicrobial groups in HA non-inpatient service . 10 4.2.2 Ten most dispensed antimicrobials in HA non-inpatient service . 10 4.2.3 Antimicrobial dispensed in HA non-inpatient service, stratified by service type ................................ 11 4.3 Overall annual dispensed quantities and percentage changes in HA inpatient service ....................................... 12 4.3.1 Five most dispensed antimicrobial groups in HA inpatient service . 13 4.3.2 Ten most dispensed antimicrobials in HA inpatient service . 14 4.3.3 Ten most dispensed antimicrobials in HA inpatient service, stratified by specialty ................................. 15 4.4 Overall annual dispensed quantities and percentage change of locally-important broad-spectrum antimicrobials in all HA services . 16 4.4.1 Locally-important broad-spectrum antimicrobial dispensed in HA inpatient service, stratified by specialty .