Flows of Selected Materials Associated with World Copper Smelting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Softening Behaviour of Lead Sinter and Slag At
Paper Title: THE ISA-YMG LEAD SMELTING PROCESS Paper Presented at: PbZn 2005 Conference, Kyoto, Japan Authors: Bill Errington & Philip Arthur, Xstrata Technology Jikun Wang & Ying Dong, Yunnan Metallurgical Group, Date of Publication: October, 2005 For further information please contact us at [email protected] www.isasmelt.com THE ISA-YMG LEAD SMELTING PROCESS Bill Errington and Philip Arthur Xstrata Technology, Level 4, 307 Queen Street,, Brisbane, Queensland 4000, Australia [email protected] Jikun Wang and Ying Dong Yunnan Metallurgical Group, Kunming,Yunnan, P. R. China ABSTRACT The Yunnan Metallurgical Group (YMG) has constructed a new lead-zinc smelting/refinery complex at Qujing in Yunnan Province, China. The lead smelting process combines an ISASMELT™ smelting furnace with a YMG designed blast furnace. The ISASMELT furnace produces lead bullion plus a high-lead slag that is cast into lump form and fed to the blast furnace. YMG installed a blast furnace in the 1950's to treat high lead slag produced by earlier silver operations. This furnace later treated sinter. For the present project YMG carried out trials at the pilot and commercial scale before deciding on the final blast furnace design. The ISASMELT furnace design was based on the operation of the Lead ISASMELT Plant at Mount Isa. This paper describes the development and the operation of both the ISASMELT furnace and the YMG blast furnace. This combination of new technology and remodelled traditional technology provides an economical solution to achieving an environmentally acceptable lead smelter. Key words: ISASMELT process, lead smelting, lead slag, lead blast furnace www.isasmelt.com 2 INTRODUCTION The new YMG lead smelter forms one part of a new greenfield lead/zinc smelting and refinery complex located at Qujing in Yunnan Province in China. -

Principles of Extractive Metallurgy Lectures Note
PRINCIPLES OF EXTRACTIVE METALLURGY B.TECH, 3RD SEMESTER LECTURES NOTE BY SAGAR NAYAK DR. KALI CHARAN SABAT DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING PARALA MAHARAJA ENGINEERING COLLEGE, BERHAMPUR DISCLAIMER This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the author for their respective teaching assignments as an additional tool for the teaching-learning process. Various sources as mentioned at the reference of the document as well as freely available material from internet were consulted for preparing this document. The ownership of the information lies with the respective author or institutions. Further, this document is not intended to be used for commercial purpose and the faculty is not accountable for any issues, legal or otherwise, arising out of use of this document. The committee faculty members make no representations or warranties with respect to the accuracy or completeness of the contents of this document and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. BPUT SYLLABUS PRINCIPLES OF EXTRACTIVE METALLURGY (3-1-0) MODULE I (14 HOURS) Unit processes in Pyro metallurgy: Calcination and roasting, sintering, smelting, converting, reduction, smelting-reduction, Metallothermic and hydrogen reduction; distillation and other physical and chemical refining methods: Fire refining, Zone refining, Liquation and Cupellation. Small problems related to pyro metallurgy. MODULE II (14 HOURS) Unit processes in Hydrometallurgy: Leaching practice: In situ leaching, Dump and heap leaching, Percolation leaching, Agitation leaching, Purification of leach liquor, Kinetics of Leaching; Bio- leaching: Recovery of metals from Leach liquor by Solvent Extraction, Ion exchange , Precipitation and Cementation process. -

Corrosion of Refractories in Lead Smelting Reactors
CORROSION OF REFRACTORIES IN LEAD SMELTING REACTORS By LINGXUAN WEI B.Sc, Wuhan University of Science & Technology, China 1986 M.Sc, University of Science & Technology Beijing, China 1997 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF APPLIED SCIENCE in THE FACULTY OF GRADUATE STUDIES DEPARTMENT OF METALS AND MATERIALS ENGINEERING We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH .UMB1A DECEMBER 2000 ©Lingxuan Wei, ZO0O UBC Special Collections - Thesis Authorisation Form http://www.library.ubc.ca/spcoll/thesauth.html In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. v 3 The University of British Columbia Vancouver, Canada Date lof 1 3/19/01 2:36 PM ABSTRACT Corrosion of refractories by slag is a complex phenomenon which, depending on the particular system, involves many processes, such as chemical wear (corrosion) and physical or mechanical wear (erosion), which may act synergistically. No single model can explain all cases of corrosion nor can it explain all corrosion mechanisms of a particular refractory in different environments, but the knowledge of the microstructure combined with the chemistry of the systems are necessary to understand the corrosion mechanism of a refractory material. -

Conflict Minerals Report for 2019, We Have Completed Our Analysis of The
Conflict Minerals Report For 2019, we have completed our analysis of the suppliers for Amazon electronic devices, fashion and apparel, and other consumer products that fall within the scope of the applicable rules (collectively, in-scope products). A majority of these suppliers certified that they: did not use gold, tin, tungsten, or tantalum in parts or components for our in-scope products; did not source these minerals from the Democratic Republic of the Congo or an adjoining country, referred to as the DRC region; or sourced these minerals from a smelter or refiner that complies with a responsible mineral sourcing validation program such as the Responsible Minerals Assurance Process. The remaining suppliers are still completing investigations of their supply chains. As discussed in the Conclusion, for 2019, we identified no suppliers that were sourcing minerals through a supply chain that benefitted armed groups in the DRC region. Pursuant to the Securities and Exchange Commission’s conflict minerals rules, we designed our due diligence on the source and chain of custody of the gold, tin, tungsten, and tantalum in our in-scope products in accordance with the OECD’s Due Diligence Guidance for Responsible Supply Chains of Minerals from Conflict-Affected and High-Risk Areas Third Edition, and the related Supplements on Tin, Tantalum and Tungsten and on Gold. We undertook the steps described below as part of our due diligence process: Establish strong company management systems We are committed to avoiding the use of minerals that have fueled conflict, and we expect our suppliers to support our efforts to identify the origin of gold, tin, tungsten, and tantalum used in products that we manufacture or contract to manufacture. -

The Mineral Industry of China in 2016
2016 Minerals Yearbook CHINA [ADVANCE RELEASE] U.S. Department of the Interior December 2018 U.S. Geological Survey The Mineral Industry of China By Sean Xun In China, unprecedented economic growth since the late of the country’s total nonagricultural employment. In 2016, 20th century had resulted in large increases in the country’s the total investment in fixed assets (excluding that by rural production of and demand for mineral commodities. These households; see reference at the end of the paragraph for a changes were dominating factors in the development of the detailed definition) was $8.78 trillion, of which $2.72 trillion global mineral industry during the past two decades. In more was invested in the manufacturing sector and $149 billion was recent years, owing to the country’s economic slowdown invested in the mining sector (National Bureau of Statistics of and to stricter environmental regulations in place by the China, 2017b, sec. 3–1, 3–3, 3–6, 4–5, 10–6). Government since late 2012, the mineral industry in China had In 2016, the foreign direct investment (FDI) actually used faced some challenges, such as underutilization of production in China was $126 billion, which was the same as in 2015. capacity, slow demand growth, and low profitability. To In 2016, about 0.08% of the FDI was directed to the mining address these challenges, the Government had implemented sector compared with 0.2% in 2015, and 27% was directed to policies of capacity control (to restrict the addition of new the manufacturing sector compared with 31% in 2015. -

2020 Conflict Minerals Report
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM SD Specialized Disclosure Report O-I GLASS, INC. (Exact name of registrant as specified in its charter) Delaware 1-9576 22-2781933 (State or other jurisdiction of (Commission (IRS Employer incorporation or organization) file number) Identification No.) One Michael Owens Way, Perrysburg, Ohio 43551 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (567) 336-5000 Mary Beth Wilkinson (567) 336-5000 (Name and telephone number, including area code, of the person to contact in connection with this report.) Check the appropriate box to indicate the rule pursuant to which this form is being filed, and provide the period to which the information in this form applies: ☒ Rule 13p-1 under the Securities Exchange Act (17 CFR 240.13p-1) for the reporting period from January 1 to December 31, 2019. Section 1 - Conflict Minerals Disclosure Item 1.01 Conflict Minerals Disclosure and Report A copy of the Conflict Minerals Report for O-I Glass, Inc. (the “Company”) is provided as Exhibit 1.01 hereto and is publicly available at www.o-i.com. The term “Company,” as used herein and unless otherwise stated or indicated by context, refers to Owens-Illinois, Inc. and its affiliates (“O-I”) prior to the Corporate Modernization (as defined below) and to O-I Glass, Inc. and its affiliates (“O-I Glass”) after the Corporate Modernization. On December 26 and 27, 2019, the Company implemented the Corporate Modernization (“Corporate Modernization”), whereby O-I Glass became the new parent entity with Owens-Illinois Group, Inc. -

Borsa Istanbul A.S. List of Refineries; Bars, Ingots and Granules of Which Can Be Traded in the Precious Metals and Precious Stones Market
BORSA ISTANBUL A.S. LIST OF REFINERIES; BARS, INGOTS AND GRANULES OF WHICH CAN BE TRADED IN THE PRECIOUS METALS AND PRECIOUS STONES MARKET GOLD REFINERIES (REFINERIES ALSO INCLUDED IN LBMA GOOD DELIVERY LIST) USA Asahi Refining USA Inc. Kennecott Utah Copper LLC Metalor USA Refining Corp. Germany Allgemeine Gold- und Silberscheideanstalt AG Aurubis AG C. Hafner GmbH + Co. KG Heimerle + Meule GmbH Australia ABC Refinery (Australia) Pty Ltd Western Australian Mint (T/A The Perth Mint) Belgium Umicore SA Business Unit Precious Metals Refining Brasil AngloGold Ashanti Mineração Ltda China Daye Nonferrous Metals Co., Ltd Great Wall Precious Metals Co., LTD. of CBPM Inner Mongolia Qiankun Gold and Silver Refinery Share Company Ltd Jiangxi Copper Company Ltd Shandong Gold Smelting Co., Ltd Shandong Zhaojin Gold and Silver Refinery Co. Ltd Sichuan Tianze Precious Metals Co., Ltd Zhongyuan Gold Smelter of Zhongjin Gold Corporation Zijin Mining Group Co. Ltd Metalor Precious Metals (Suzhou) Limited Indonesia PT Aneka Tambang (Persero) Tbk (Logam Mulia) Philippines Bangko Sentral ng Pilipinas (Central Bank of the Philippines) South Africa Rand Refinery (Pty) Ltd South Korea LS Nikko Copper Inc India MMTC-PAMP India Pvt. Ltd Hong Kong Heraeus Ltd Hong Kong Metalor Technologies (Hong Kong) Ltd Spain Sempsa Joyeria Plateria SA Sweden Boliden Commercial AB Switzerland Argor-Heraeus SA Metalor Technologies SA Pamp SA PX Précinox SA Valcambi S.A. Italy Chimet S.p.A. T.C.A S.p.A Italpreziosi S.p. A Japan Asahi Pretec Corp Ishifuku Metal Industry Co Ltd Japan Mint JX Nippon Mining & Metals Co., Ltd Matsuda Sangyo Co., Ltd Mitsubishi Materials Corp. -

Xstrata Technology Update Edition 13 – April 2012 Building Plants That Work
xstrata technology update Edition 13 – April 2012 Building plants that work You have to get a lot of things it takes another operator to get them right to build a plant that works. right. Someone who has lived through the problems, had to do the maintenance, operated during a midnight power Of course the big picture must be right – doing the right project, in the right place, failure, cleaned up the spill. Someone at the right time. who has “closed the loop” on previous designs; lived with previous decisions After that, the devil is in the detail. You and improved them, over and over. need a sound design, good execution, good commissioning, and ongoing This is why Xstrata Technology provides support after commissioning. You need a technology “package”. Just as a car to operate and maintain your plant in is more than an engine, technology is the long run, long after the construction more than a single piece of equipment. company has left. That’s when all the Technology is a system. All the elements “little” details become important – how of the system have to work with each easy is it to operate, how good is the other and with the people in the plant. maintenance access, what happens in We want our cars designed by people a power failure, where are the spillage who love cars and driving. So should points and how do we clean them our plants be designed by people with up? Are the instruments reliable and experience and passion to make each is the process control strategy robust one work better than the last. -

China and Global Markets: Copper Supply Chain Sustainable Development
ChinaChina andand GlobalGlobal Markets:Markets: CopperCopper SupplySupply ChainChain SustainableSustainable DevelopmentDevelopment A Life Cycle Assessment Study Martin Streicher-Porte, Empa HansMartin Jörg Streicher Althaus,-Porte, Empa Empa Hans Jörg Althaus, Empa February 2010 February 2010 Click here to enter text. 2 © 2011 International Institute for Sustainable Development (IISD) China and Global Published by the International Institute for Markets: Sustainable Development Copper Supply Chain IISD contributes to sustainable development by advancing policy recommendations on international Sustainable trade and investment, economic policy, climate change and energy, measurement and assessment, and natural resources management, and the enabling role Development: of communication technologies in these areas. We report on international negotiations and disseminate A Life Cycle knowledge gained through collaborative projects, resulting in more rigorous research, capacity building Assessment Study in developing countries, better networks spanning the North and the South, and better global connections among researchers, practitioners, citizens and policy- makers. Martin Streicher-Porte IISD’s vision is better living for all—sustainably; its Hans-Jörg Althaus mission is to champion innovation, enabling societies Empa—Materials Science and to live sustainably. IISD is registered as a charitable Technology organization in Canada and has 501(c)(3) status in the Technology and Society Laboratory United States. IISD receives core operating support Lerchenfeldstrasse 5 from the Government of Canada, provided through the Canadian International Development Agency CH-9014 St. Gallen, Switzerland (CIDA), the International Development Research Phone +41 (0)71 274 74 74 Centre (IDRC) and Environment Canada, and from Fax +41 (0)71 274 74 62 the Province of Manitoba. The Institute receives [email protected] project funding from numerous governments inside and outside Canada, United Nations agencies, [email protected] foundations and the private sector. -
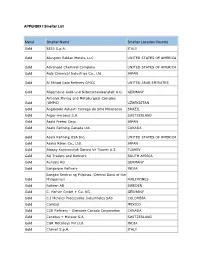
2020 Appendix I Smelter List
APPENDIX I Smelter List Metal Smelter Name Smelter Location Country Gold 8853 S.p.A. ITALY Gold Abington Reldan Metals, LLC UNITED STATES OF AMERICA Gold Advanced Chemical Company UNITED STATES OF AMERICA Gold Aida Chemical Industries Co., Ltd. JAPAN Gold Al Etihad Gold Refinery DMCC UNITED ARAB EMIRATES Gold Allgemeine Gold-und Silberscheideanstalt A.G. GERMANY Almalyk Mining and Metallurgical Complex Gold (AMMC) UZBEKISTAN Gold AngloGold Ashanti Corrego do Sitio Mineracao BRAZIL Gold Argor-Heraeus S.A. SWITZERLAND Gold Asahi Pretec Corp. JAPAN Gold Asahi Refining Canada Ltd. CANADA Gold Asahi Refining USA Inc. UNITED STATES OF AMERICA Gold Asaka Riken Co., Ltd. JAPAN Gold Atasay Kuyumculuk Sanayi Ve Ticaret A.S. TURKEY Gold AU Traders and Refiners SOUTH AFRICA Gold Aurubis AG GERMANY Gold Bangalore Refinery INDIA Bangko Sentral ng Pilipinas (Central Bank of the Gold Philippines) PHILIPPINES Gold Boliden AB SWEDEN Gold C. Hafner GmbH + Co. KG GERMANY Gold C.I Metales Procesados Industriales SAS COLOMBIA Gold Caridad MEXICO Gold CCR Refinery - Glencore Canada Corporation CANADA Gold Cendres + Metaux S.A. SWITZERLAND Gold CGR Metalloys Pvt Ltd. INDIA Gold Chimet S.p.A. ITALY Gold Chugai Mining JAPAN Gold Daye Non-Ferrous Metals Mining Ltd. CHINA Gold Degussa Sonne / Mond Goldhandel GmbH GERMANY Gold DODUCO Contacts and Refining GmbH GERMANY Gold Dowa JAPAN Gold DSC (Do Sung Corporation) KOREA, REPUBLIC OF Gold Eco-System Recycling Co., Ltd. East Plant JAPAN Gold Eco-System Recycling Co., Ltd. North Plant JAPAN Gold Eco-System Recycling Co., Ltd. West Plant JAPAN Gold Emirates Gold DMCC UNITED ARAB EMIRATES Gold GCC Gujrat Gold Centre Pvt. -

The Mineral Industry of China in 2007
2007 Minerals Yearbook CHINA U.S. Department of the Interior December 2009 U.S. Geological Survey THE MINERAL INDUS T RY OF CHINA By Pui-Kwan Tse After three decades of economic development, China probably have some impact on the global commodity prices has become one of the leading economic and trade powers (Batson and King, 2008). in the world, one of the top destinations for foreign direct investment, and an export destination of choice. During the Minerals in the National Economy past several years, the Government’s economic policy was to prevent economic slowdown and fight inflation. In 2007, China is rich in mineral resources and was the world’s China’s economic growth rate was 11.9% and represented the leading producer of aluminum, antimony, barite, bismuth, coal, fifth consecutive year of double-digit expansion. Industrial fluorspar, gold, graphite, iron and steel, lead, phosphate rock, production increased by 18.5% compared with that of 2006. rare earths, talc, tin, tungsten, and zinc in 2007. It ranked among The consumer price index (a measurement of inflation) rose to the top three countries in the world in the production of many a decade high of 4.8%. To prevent economic “overheating,” the other mineral commodities. China was the leading exporter Government raised interest rates and the People’s Bank of China of antimony, barite, coal, fluorspar, graphite, rare earths, and (the central bank) required commercial banks to increase the tungsten in the world. The country’s demand for chromium, reserve-requirement ratio 11 times to 11.5% at yearend 2007. -

The Advanced Mining Technologies and Its Impact on the Australian Nonferrous Minerals Industry
Paper Title: PROVEN TECHNOLOGIES FROM XSTRATA AND THEIR APPLICATIONS FOR COPPER SMELTING AND REFINING IN CHINA Paper Presented at: Hainan Conference, China Authors: Mingwei Gao, Philip Arthur and Nigel Aslin, Xstrata Technology Date of Publication: 2004 For further information please contact us at [email protected] www.isasmelt.com PROVEN TECHNOLOGIES FROM XSTRATA AND THEIR APPLICATIONS FOR COPPER SMELTING AND REFINING IN CHINA Mingwei Gao, Philip Arthur and Nigel Aslin Xstrata Technology, Australia 1.0 INTRODUCTION The world’s non-ferrous industry has achieved significant improvements in efficiency in the last 20 years owing to the advanced technologies such as ISASMELT™ and ISA PROCESS that have been invented and developed at Mount Isa Mines in Australia,. A number of large nonferrous mining operations in the world are still in business today largely because of the commercial benefits that resulted from applying these technologies. ISASMELT and ISA PROCESS technologies are marketed worldwide by Xstrata Technology, a division of the Xstrata plc group of companies, which was formed upon Xstrata’s takeover of MIM Holdings Limited in mid 2003. Xstrata PLC is a diversified international mining company and has operations in Australia, the United Kingdom, Germany, Spain, South Africa, Chile, and Argentina with around 20,000 employees worldwide. Major products are copper, lead, zinc, silver, coal, ferrochrome, and ferrovanadium. In addition to these commodities, an integral part of the group is their independent process technology business – Xstrata Technology. Xstrata group companies have a long record of developing process technologies for in-house use and for sale to external clients. 2.0 ISASMELT™ TECHNOLOGY ISASMELT is a modern bath-smelting process for the production of non-ferrous metals.