Condoms and Stds: Fact Sheet for Public Health Personnel Consistent and Correct Use of Latex Condoms Can Reduce (Though Not Eliminate) the Risk of STD Transmission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

Commentary Disease Transmission Dynamics and the Evolution
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 800–801, February 1999 Commentary Disease transmission dynamics and the evolution of antibiotic resistance in hospitals and communal settings Simon A. Levin*† and Viggo Andreasen‡ *Department of Ecology and Evolutionary Biology, Eno Hall, Princeton University, Princeton NJ 08544; and ‡Department of Mathematics, Roskilde University, DK-4000 Roskilde, Denmark Despite the tremendous benefits of antibiotics for dealing with sally, exercising little influence on their host under normal a wide range of pathogens, there is by now little doubt that conditions. Antibiotics are typically introduced to treat the their indiscriminate use has led to the emergence of novel true pathogens, and for them the problem of resistance resistant strains and a frightening new set of threats to public introduces questions concerning the length and intensity of health. Hospitals and other community settings provide an treatment, multidrug strategies, and patient compliance. (See, especially fertile ground for the spread of those types; in for example, refs. 2–4). Commensals provide a different sort particular, the recent emergence and proliferation of bacteria of problem, however. Under normal conditions, they live in resistant to both methicillin and vancomycin has engendered such places as the skin or upper respiratory tract, causing little serious concern, threatening the effectiveness of the last or no harm. On occasion, however, they become translocated available options for treatment of potentially fatal Staphylo- to sites that are normally sterile, such as the blood or lungs, coccus strains. where they may have serious harmful effects (6). Many viru- It seems clear that a considered and comprehensive strategy lence factors, for example in Staphylococcus aureus, are carried for antibiotic use is essential, permitting the intelligent de- on plasmids, and can be exchanged among different strains. -

Valacyclovir Reduced Genital Herpes Transmission in Couples Discordant for Herpes Simplex Virus Type 2 Infection Corey L, Wald A, Patel R, Et Al
146 THERAPEUTICS Evid Based Med: first published as 10.1136/ebm.9.5.146 on 30 September 2004. Downloaded from Valacyclovir reduced genital herpes transmission in couples discordant for herpes simplex virus type 2 infection Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004;350:11–20. Clinical impact ratings GP/FP/Primary care wwwwwwq Infectious diseases wwwwwqq ............................................................................................................................... In heterosexual couples who are serologically discordant for herpes simplex virus type 2 (HSV-2) infection, does once Q daily valacyclovir reduce the sexual transmission of genital herpes? METHODS MAIN RESULTS The table shows the results. Design: randomised placebo controlled trial. CONCLUSION In heterosexual couples discordant for herpes simplex virus type 2 infection, valacyclovir reduced transmission of infection. Allocation: concealed.* Commentary Blinding: blinded {participants, healthcare providers, data revious studies have shown that antiviral drugs can reduce the collectors, data analysts, and outcome assessors}À.* frequency of recurrence and subclinical shedding of viral particles.1 Follow up period: 8 months. P This important, large, well designed trial by Corey et al is the first to show an effect on the transmission of HSV-2 infection to an uninfected partner. In this study, the reduction in transmission was limited; 62 partners had Setting: 96 sites in the US, Canada, Europe, Latin America, and to take the drug daily for 8 months to prevent 1 infection, and 57 partners Australia. had to take the drug daily to prevent overall acquisition of HSV-2 infection. 2 independent predictors were found in addition to the drugs: Participants: 1498 couples >18 years who were women as the susceptible partners (hazard ratio [HR] 3.3) and duration immunocompetent, heterosexual, monogamous, in good health, of HSV-2 infection ,2 years (HR 2.9). -

2012 Case Definitions Infectious Disease
Arizona Department of Health Services Case Definitions for Reportable Communicable Morbidities 2012 TABLE OF CONTENTS Definition of Terms Used in Case Classification .......................................................................................................... 6 Definition of Bi-national Case ............................................................................................................................................. 7 ------------------------------------------------------------------------------------------------------- ............................................... 7 AMEBIASIS ............................................................................................................................................................................. 8 ANTHRAX (β) ......................................................................................................................................................................... 9 ASEPTIC MENINGITIS (viral) ......................................................................................................................................... 11 BASIDIOBOLOMYCOSIS ................................................................................................................................................. 12 BOTULISM, FOODBORNE (β) ....................................................................................................................................... 13 BOTULISM, INFANT (β) ................................................................................................................................................... -

Sexually Transmitted Diseases/Infections Chancroid
Sexually Transmitted Diseases/Infections Chancroid Chancroid is a bacterial infection caused by Haemophilus ducreyi. It is spread by sexual contact and results in genital ulcers. Chancroid is a reportable genital ulcer condition that is rarely seen in North Carolina. When infection does occur, it is usually associated with sporadic outbreaks. Chancroid, as well as genital herpes and syphilis, is a risk factor in the transmission of HIV infection. Chancroid lesions may be difficult to distinguish from ulcers caused by genital herpes or syphilis. A physician must therefore diagnose the infection by excluding other diseases with similar symptoms. The combination of a painful genital ulcer and tender suppurative inguinal adenopathy suggests the diagnosis of chancroid. A probable diagnosis of chancroid, for both clinical and surveillance purposes, can be made if all of the following criteria are met: 1) the patient has one or more painful genital ulcers; 2) the patient has no evidence of T. pallidum infection by darkfield examination of ulcer exudate or by a serologic test for syphilis performed at least 7 days after onset of ulcers; 3) the clinical presentation, appearance of genital ulcers and, if present, regional lymphadenopathy are typical for chancroid; and 4) a test for HSV performed on the ulcer exudate is negative. A definitive diagnosis of chancroid requires the identification of H. ducreyi on special culture media that is not widely available from commercial sources; even when these media are used, sensitivity is less than 80 percent. No FDA- cleared PCR test for H. ducreyi is available in the United States, but such testing can be performed by clinical laboratories that have developed their own PCR test and have conducted a CLIA verification study. -

Reportable Disease Surveillance in Virginia, 2013
Reportable Disease Surveillance in Virginia, 2013 Marissa J. Levine, MD, MPH State Health Commissioner Report Production Team: Division of Surveillance and Investigation, Division of Disease Prevention, Division of Environmental Epidemiology, and Division of Immunization Virginia Department of Health Post Office Box 2448 Richmond, Virginia 23218 www.vdh.virginia.gov ACKNOWLEDGEMENT In addition to the employees of the work units listed below, the Office of Epidemiology would like to acknowledge the contributions of all those engaged in disease surveillance and control activities across the state throughout the year. We appreciate the commitment to public health of all epidemiology staff in local and district health departments and the Regional and Central Offices, as well as the conscientious work of nurses, environmental health specialists, infection preventionists, physicians, laboratory staff, and administrators. These persons report or manage disease surveillance data on an ongoing basis and diligently strive to control morbidity in Virginia. This report would not be possible without the efforts of all those who collect and follow up on morbidity reports. Divisions in the Virginia Department of Health Office of Epidemiology Disease Prevention Telephone: 804-864-7964 Environmental Epidemiology Telephone: 804-864-8182 Immunization Telephone: 804-864-8055 Surveillance and Investigation Telephone: 804-864-8141 TABLE OF CONTENTS INTRODUCTION Introduction ......................................................................................................................................1 -
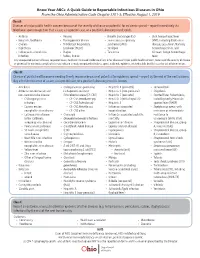
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Potential for Transmission of Antimicrobial Resistance In
Report of the Scientific Committee of the Food Safety Authority of Ireland 2015 Potential for Transmission of Antimicrobial Resistance in the Food Chain Report of the Scientific Committee of the Food Safety Authority of Ireland Potential for Transmission of Antimicrobial Resistance in the Food Chain Published by: Food Safety Authority of Ireland Abbey Court, Lower Abbey St Dublin 1 DO1 W2H4 Tel: +353 1 8171300 Fax: +353 1 8171301 [email protected] www.fsai.ie © FSAI 2015 Applications for reproduction should be made to the FSAI Information Unit ISBN 978-1-910348-01-7 Report of the Scientific Committee of the Food Safety Authority of Ireland Potential for Transmission of Antimicrobial Resistance in the Food Chain CONTENTS FOREWORD 4 ACKNOWLEDGEMENTS 5 ABBREVIATIONS 6 EXECUTIVE SUMMARY 7 CHAPTER 1. INTRODUCTION 10 1.1 The Scale of the Antimicrobial Resistance Problem ...................10 1.2 Human and Animals – The ‘One Health’ Concept.....................11 1.3 The Background to the Antimicrobial Resistance Problem . .11 1.4 Genetic Diversity, Genetic Determinants and Change in Bacteria . 14 1.5 Selection for Antimicrobial Resistance...............................15 1.6 Surveillance of Antimicrobial Resistance in Ireland and Europe . .15 1.7 Prudent Use of Antimicrobials – Definition . 15 1.8 Surveillance of Antimicrobial Use in Ireland and Europe ...............16 1.9 Antimicrobials and the Food Chain . .16 1.10 Antimicrobial-resistant Bacteria and the Food Chain ..................16 1.11 Summary . .17 1.12 Chapter Bibliography..............................................17 CHAPTER 2. TERMS OF REFERENCE 20 2.1 Chapter Bibliography..............................................20 1 of 58 Report of the Scientific Potential for Transmission of Committee of the Food Safety Authority of Ireland Antimicrobial Resistance in the Food Chain CHAPTER 3. -

Sex Education and Condom Distribution: John, Susan, Parents, and Schools Jeffrey F
Notre Dame Journal of Law, Ethics & Public Policy Volume 10 Article 7 Issue 2 Symposium on Law and the Family 1-1-2012 Sex Education and Condom Distribution: John, Susan, Parents, and Schools Jeffrey F. Caruso Follow this and additional works at: http://scholarship.law.nd.edu/ndjlepp Recommended Citation Jeffrey F. Caruso, Sex Education and Condom Distribution: John, Susan, Parents, and Schools, 10 Notre Dame J.L. Ethics & Pub. Pol'y 663 (1996). Available at: http://scholarship.law.nd.edu/ndjlepp/vol10/iss2/7 This Note is brought to you for free and open access by the Notre Dame Journal of Law, Ethics & Public Policy at NDLScholarship. It has been accepted for inclusion in Notre Dame Journal of Law, Ethics & Public Policy by an authorized administrator of NDLScholarship. For more information, please contact [email protected]. STUDENT NOTE SEX EDUCATION AND CONDOM DISTRIBUTION: JOHN, SUSAN, PARENTS, AND SCHOOLS JEFFREY F. CARuso* I. INTRODUCTION John, a high school freshman, walked down the corridor, passed several classrooms, and entered the boys' restroom on a Friday afternoon. He fished into his pocket for a few quarters, inserted them into the vending machine, and pulled out a con- dom. As he completed the transaction, he reminded himself of how glad he was that his parents did not know how he spends his extra lunch money. He knew he'd still have to concoct another story for when they'd ask him where he was going that night, but he quickly assured himself that he'd have plenty of time to think of something. -

Zoonotic Diseases Fact Sheet
ZOONOTIC DISEASES FACT SHEET s e ion ecie s n t n p is ms n e e s tio s g s m to a a o u t Rang s p t tme to e th n s n m c a s a ra y a re ho Di P Ge Ho T S Incub F T P Brucella (B. Infected animals Skin or mucous membrane High and protracted (extended) fever. 1-15 weeks Most commonly Antibiotic melitensis, B. (swine, cattle, goats, contact with infected Infection affects bone, heart, reported U.S. combination: abortus, B. suis, B. sheep, dogs) animals, their blood, tissue, gallbladder, kidney, spleen, and laboratory-associated streptomycina, Brucellosis* Bacteria canis ) and other body fluids causes highly disseminated lesions bacterial infection in tetracycline, and and abscess man sulfonamides Salmonella (S. Domestic (dogs, cats, Direct contact as well as Mild gastroenteritiis (diarrhea) to high 6 hours to 3 Fatality rate of 5-10% Antibiotic cholera-suis, S. monkeys, rodents, indirect consumption fever, severe headache, and spleen days combination: enteriditis, S. labor-atory rodents, (eggs, food vehicles using enlargement. May lead to focal chloramphenicol, typhymurium, S. rep-tiles [especially eggs, etc.). Human to infection in any organ or tissue of the neomycin, ampicillin Salmonellosis Bacteria typhi) turtles], chickens and human transmission also body) fish) and herd animals possible (cattle, chickens, pigs) All Shigella species Captive non-human Oral-fecal route Ranges from asymptomatic carrier to Varies by Highly infective. Low Intravenous fluids primates severe bacillary dysentery with high species. 16 number of organisms and electrolytes, fevers, weakness, severe abdominal hours to 7 capable of causing Antibiotics: ampicillin, cramps, prostration, edema of the days. -

Evolution of Disease Transmission During the COVID-19
www.nature.com/scientificreports OPEN Evolution of disease transmission during the COVID‑19 pandemic: patterns and determinants Jie Zhu & Blanca Gallego* Epidemic models are being used by governments to inform public health strategies to reduce the spread of SARS‑CoV‑2. They simulate potential scenarios by manipulating model parameters that control processes of disease transmission and recovery. However, the validity of these parameters is challenged by the uncertainty of the impact of public health interventions on disease transmission, and the forecasting accuracy of these models is rarely investigated during an outbreak. We ftted a stochastic transmission model on reported cases, recoveries and deaths associated with SARS‑CoV‑2 infection across 101 countries. The dynamics of disease transmission was represented in terms of the daily efective reproduction number ( Rt ). The relationship between public health interventions and Rt was explored, frstly using a hierarchical clustering algorithm on initial Rt patterns, and secondly computing the time‑lagged cross correlation among the daily number of policies implemented, Rt , and daily incidence counts in subsequent months. The impact of updating Rt every time a prediction is made on the forecasting accuracy of the model was investigated. We identifed 5 groups of countries with distinct transmission patterns during the frst 6 months of the pandemic. Early adoption of social distancing measures and a shorter gap between interventions were associated with a reduction on the duration of outbreaks. The lagged correlation analysis revealed that increased policy volume was associated with lower future Rt (75 days lag), while a lower Rt was associated with lower future policy volume (102 days lag).