Orica Richmond Vale Biodiversity Offset Area Monitoring Report – 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Native Orchid Society South Australia
Journal of the Native Orchid Society of South Australia Inc PRINT POST APPROVED VOLUME 25 NO. 11 PP 54366200018 DECEMBER 2001 NATIVE ORCHID SOCIETY OF SOUTH AUSTRALIA POST OFFICE BOX 565 UNLEY SOUTH AUSTRALIA 5061 The Native Orchid Society of South Australia promotes the conservation of orchids through the preservation of natural habitat and through cultivation. Except with the documented official representation from the Management Committee no person is authorised to represent the society on any matter. All native orchids are protected plants in the wild. Their collection without written Government permit is illegal. PRESIDENT: SECRETARY: Bill Dear Cathy Houston Telephone: 82962111 Telephone: 8356 7356 VICE-PRESIDENT David Pettifor Tel. 014095457 COMMITTEE David Hirst Thelma Bridle Bob Bates Malcolm Guy EDITOR: TREASURER Gerry Carne Iris Freeman 118 Hewitt Avenue Toorak Gardens SA 5061 Telephone/Fax 8332 7730 E-mail [email protected] LIFE MEMBERS Mr R. Hargreaves Mr G. Carne Mr L. Nesbitt Mr R. Bates Mr R. Robjohns Mr R Shooter Mr D. Wells Registrar of Judges: Reg Shooter Trading Table: Judy Penney Field Trips & Conservation: Thelma Bridle Tel. 83844174 Tuber Bank Coordinator: Malcolm Guy Tel. 82767350 New Members Coordinator David Pettifor Tel. 0416 095 095 PATRON: Mr T.R.N. Lothian The Native Orchid Society of South Australia Inc. while taking all due care, take no responsibility for the loss, destruction or damage to any plants whether at shows, meetings or exhibits. Views or opinions expressed by authors of articles within this Journal do not necessarily reflect the views or opinions of the Management. We condones the reprint of any articles if acknowledgement is given. -

The Native Vegetation of the Nattai and Bargo Reserves
The Native Vegetation of the Nattai and Bargo Reserves Project funded under the Central Directorate Parks and Wildlife Division Biodiversity Data Priorities Program Conservation Assessment and Data Unit Conservation Programs and Planning Branch, Metropolitan Environmental Protection and Regulation Division Department of Environment and Conservation ACKNOWLEDGMENTS CADU (Central) Manager Special thanks to: Julie Ravallion Nattai NP Area staff for providing general assistance as well as their knowledge of the CADU (Central) Bioregional Data Group area, especially: Raf Pedroza and Adrian Coordinator Johnstone. Daniel Connolly Citation CADU (Central) Flora Project Officer DEC (2004) The Native Vegetation of the Nattai Nathan Kearnes and Bargo Reserves. Unpublished Report. Department of Environment and Conservation, CADU (Central) GIS, Data Management and Hurstville. Database Coordinator This report was funded by the Central Peter Ewin Directorate Parks and Wildlife Division, Biodiversity Survey Priorities Program. Logistics and Survey Planning All photographs are held by DEC. To obtain a Nathan Kearnes copy please contact the Bioregional Data Group Coordinator, DEC Hurstville Field Surveyors David Thomas Cover Photos Teresa James Nathan Kearnes Feature Photo (Daniel Connolly) Daniel Connolly White-striped Freetail-bat (Michael Todd), Rock Peter Ewin Plate-Heath Mallee (DEC) Black Crevice-skink (David O’Connor) Aerial Photo Interpretation Tall Moist Blue Gum Forest (DEC) Ian Roberts (Nattai and Bargo, this report; Rainforest (DEC) Woronora, 2003; Western Sydney, 1999) Short-beaked Echidna (D. O’Connor) Bob Wilson (Warragamba, 2003) Grey Gum (Daniel Connolly) Pintech (Pty Ltd) Red-crowned Toadlet (Dave Hunter) Data Analysis ISBN 07313 6851 7 Nathan Kearnes Daniel Connolly Report Writing and Map Production Nathan Kearnes Daniel Connolly EXECUTIVE SUMMARY This report describes the distribution and composition of the native vegetation within and immediately surrounding Nattai National Park, Nattai State Conservation Area and Bargo State Conservation Area. -

Bush Foods and Fibres
Australian Plants Society NORTH SHORE GROUP Ku-ring-gai Wildflower Garden Bush foods and fibres • Plant-based bush foods, medicines and poisons can come from nectar, flowers, fruit, leaves, bark, stems, sap and roots. • Plants provide fibres and materials for making many items including clothes, cords, musical instruments, shelters, tools, toys and weapons. • A fruit is the seed-bearing structure of a plant. • Do not eat fruits that you do not know to be safe to eat. Allergic reactions or other adverse reactions could occur. • We acknowledge the Traditional Custodians of this land and pay our respects to the Elders both past, present and future for they hold the memories, traditions, culture and hope of their people. Plants as food: many native plants must be processed before they are safe to eat. Flowers, nectar, pollen, Sugars, vitamins, honey, lerps (psyllid tents) minerals, starches, manna (e.g. Ribbon Gum proteins & other nutrients Eucalyptus viminalis exudate), gum (e.g. Acacia lerp manna decurrens) Fruit & seeds Staple foods Carbohydrates (sugars, starches, fibre), proteins, fats, vitamins Leaves, stalks, roots, apical Staple foods Carbohydrates, protein, buds minerals Plants such as daisies, lilies, orchids and vines Tubers, rhyzomes were a source of starchy tubers known as Carbohydrate, fibre, yams. The yam daisy Microseris lanceolata protein, vitamins, (Asteraceae) was widespread in inland NSW minerals and other states. The native yam Dioscorea transversa grows north from Stanwell Tops into Qld and Northern Territory and can be eaten raw or roasted as can those of Trachymene incisa. 1 Plant Description of food Other notes Acacia Wattle seed is a rich source of iron, Saponins and tannins and other essential elements. -

Australian Native Plants Society Canberra Region(Inc)
AUSTRALIAN NATIVE PLANTS SOCIETY CANBERRA REGION (INC) Journal Vol. 17 No. 4 December 2012 ISSN 1447-1507 Print Post Approved PP299436/00143 Contents ANPS Canberra Region Report 1 Whose Bean genus is that? 3 Winter Walks 6 Signs renewal for Frost Hollow to Forest Walk 16 Touga Road Touring 21 Study Group Snippets 25 Acacia Study Group Field Trip 27 ANPSA Study Groups 34 ANPS contacts and membership details inside back cover Cover: Correa reflexa, Kambah Pool, North; Photo: Martin Butterfield Journal articles The deadline dates for submissions are 1 February The Journal is a forum for the exchange of members' (March), 1 May (June), 1 August (September) and and others' views and experiences of gardening with, 1 November (December). Send articles or photos to: propagating and conserving Australian plants. Journal Editor All contributions, however short, are welcome. Gail Ritchie Knight Contributions may be typed or handwritten, and 1612 Sutton Road accompanied by photographs and drawings. Sutton NSW 2620 e-mail: [email protected] Submit photographs as either electronic files, tel: 0416 097 500 such as JPGs, or prints. Please enclose a stamped, self-addressed envelope if you would like your prints Paid advertising is available in this Journal. Details returned. If possible set your digital camera to take from the Editor. high resolution photos. If photos cannot be emailed, Society website: http://nativeplants-canberra.asn.au make a CD and send it by post. If you have any Printed by Elect Printing, Fyshwick, ACT queries please contact the editor http://www.electprinting.com.au/ Original text may be reprinted, unless otherwise indicated, provided an acknowledgement for the source is given. -
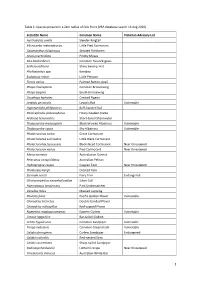
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Pollination Ecology and Evolution of Epacrids
Pollination Ecology and Evolution of Epacrids by Karen A. Johnson BSc (Hons) Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania February 2012 ii Declaration of originality This thesis contains no material which has been accepted for the award of any other degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Karen A. Johnson Statement of authority of access This thesis may be made available for copying. Copying of any part of this thesis is prohibited for two years from the date this statement was signed; after that time limited copying is permitted in accordance with the Copyright Act 1968. Karen A. Johnson iii iv Abstract Relationships between plants and their pollinators are thought to have played a major role in the morphological diversification of angiosperms. The epacrids (subfamily Styphelioideae) comprise more than 550 species of woody plants ranging from small prostrate shrubs to temperate rainforest emergents. Their range extends from SE Asia through Oceania to Tierra del Fuego with their highest diversity in Australia. The overall aim of the thesis is to determine the relationships between epacrid floral features and potential pollinators, and assess the evolutionary status of any pollination syndromes. The main hypotheses were that flower characteristics relate to pollinators in predictable ways; and that there is convergent evolution in the development of pollination syndromes. -

Existing Environment
Environmental Impact Assessment Report and Site Based Management Plan Proposed Development of Cherrabah Resort, Cherribah Cherry Gully Road, Elbow Valley Qld Existing Environment 4.1 Desktop Review Results Results from the desktop review are provided below. 4.1.1 Geology and Soil The geology of the site is described as Stanthorpe Adamellite (GSQ, 1980). The Stanthorpe Adamellite covers a vast area from about 10 km south of Warwick and 12 km west of Killarney, to Tamworth (NSW), and extending about 400 km to the west. It is part of the New England Batholith which was emplaced in two major stages during the Upper Carboniferous and later during the Upper Permian and Triassic (Rockwater, 2009). It is believed that the second stage of emplacement may have intruded the first stage, in the area near the resort, producing an extensional regime and leading to the development of the open fracture systems on the site (Rockwater, 2009). The Stanthorpe Adamellite typically comprises high potassium hornblende-biotite granite (Bryant 2004 in Rockwater, 2009). It is relatively competent, resistant to weathering and tectonically stable, resulting in large areas of massive granite outcrop and large stacked ‘tors’ (Rockwater, 2009). Drilling has revealed hard fresh granite below 20 metres depth with little indication of significant fracturing (Rockwater, 2009). In the lower lying areas immediately surrounding the resort the Stanthorpe Adamellite is highly weathered and forms rolling hills, with sandy clay soils and sandy alluvium in creeks (Rockwater, 2009). The areas of higher topographic relief to the south and east of the resort comprise fresh granite outcrop and granite ‘tors (Rockwater, 2009). -

Table of Contents Below) with Family Name Provided
1 Australian Plants Society Plant Table Profiles – Sutherland Group (updated August 2021) Below is a progressive list of all cultivated plants from members’ gardens and Joseph Banks Native Plants Reserve that have made an appearance on the Plant Table at Sutherland Group meetings. Links to websites are provided for the plants so that further research can be done. Plants are grouped in the categories of: Trees and large shrubs (woody plants generally taller than 4 m) Medium to small shrubs (woody plants from 0.1 to 4 m) Ground covers or ground-dwelling (Grasses, orchids, herbaceous and soft-wooded plants, ferns etc), as well as epiphytes (eg: Platycerium) Vines and scramblers Plants are in alphabetical order by botanic names within plants categories (see table of contents below) with family name provided. Common names are included where there is a known common name for the plant: Table of Contents Trees and Large shrubs........................................................................................................................... 2 Medium to small shrubs ...................................................................................................................... 23 Groundcovers and other ground‐dwelling plants as well as epiphytes. ............................................ 64 Vines and Scramblers ........................................................................................................................... 86 Sutherland Group http://sutherland.austplants.com.au 2 Trees and Large shrubs Acacia decurrens -

The First Chloroplast Genome Sequence of Boswellia Sacra, a Resin-Producing Plant in Oman
RESEARCH ARTICLE The First Chloroplast Genome Sequence of Boswellia sacra, a Resin-Producing Plant in Oman Abdul Latif Khan1, Ahmed Al-Harrasi1*, Sajjad Asaf2, Chang Eon Park2, Gun-Seok Park2, Abdur Rahim Khan2, In-Jung Lee2, Ahmed Al-Rawahi1, Jae-Ho Shin2* 1 UoN Chair of Oman's Medicinal Plants & Marine Natural Products, University of Nizwa, Nizwa, Oman, 2 School of Applied Biosciences, Kyungpook National University, Daegu, Republic of Korea a1111111111 * [email protected] (AAH); [email protected] (JHS) a1111111111 a1111111111 a1111111111 Abstract a1111111111 Boswellia sacra (Burseraceae), a keystone endemic species, is famous for the production of fragrant oleo-gum resin. However, the genetic make-up especially the genomic informa- tion about chloroplast is still unknown. Here, we described for the first time the chloroplast OPEN ACCESS (cp) genome of B. sacra. The complete cp sequence revealed a circular genome of 160,543 Citation: Khan AL, Al-Harrasi A, Asaf S, Park CE, bp size with 37.61% GC content. The cp genome is a typical quadripartite chloroplast struc- Park G-S, Khan AR, et al. (2017) The First ture with inverted repeats (IRs 26,763 bp) separated by small single copy (SSC; 18,962 bp) Chloroplast Genome Sequence of Boswellia sacra, and large single copy (LSC; 88,055 bp) regions. De novo assembly and annotation showed a Resin-Producing Plant in Oman. PLoS ONE 12 the presence of 114 unique genes with 83 protein-coding regions. The phylogenetic analysis (1): e0169794. doi:10.1371/journal.pone.0169794 revealed that the B. sacra cp genome is closely related to the cp genome of Azadirachta Editor: Xiu-Qing Li, Agriculture and Agri-Food indica and Citrus sinensis, while most of the syntenic differences were found in the non-cod- Canada, CANADA ing regions. -

The Vegetation of the Western Blue Mountains Including the Capertee, Coxs, Jenolan & Gurnang Areas
Department of Environment and Conservation (NSW) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas Volume 1: Technical Report Hawkesbury-Nepean CMA CATCHMENT MANAGEMENT AUTHORITY The Vegetation of the Western Blue Mountains (including the Capertee, Cox’s, Jenolan and Gurnang Areas) Volume 1: Technical Report (Final V1.1) Project funded by the Hawkesbury – Nepean Catchment Management Authority Information and Assessment Section Metropolitan Branch Environmental Protection and Regulation Division Department of Environment and Conservation July 2006 ACKNOWLEDGMENTS This project has been completed by the Special thanks to: Information and Assessment Section, Metropolitan Branch. The numerous land owners including State Forests of NSW who allowed access to their Section Head, Information and Assessment properties. Julie Ravallion The Department of Natural Resources, Forests NSW and Hawkesbury – Nepean CMA for Coordinator, Bioregional Data Group comments on early drafts. Daniel Connolly This report should be referenced as follows: Vegetation Project Officer DEC (2006) The Vegetation of the Western Blue Mountains. Unpublished report funded by Greg Steenbeeke the Hawkesbury – Nepean Catchment Management Authority. Department of GIS, Data Management and Database Environment and Conservation, Hurstville. Coordination Peter Ewin Photos Kylie Madden Vegetation community profile photographs by Greg Steenbeeke Greg Steenbeeke unless otherwise noted. Feature cover photo by Greg Steenbeeke. All Logistics -

Appendix 3 Section 5A Assessments “Seven Part Tests”
APPENDIX 3 SECTION 5A ASSESSMENTS “SEVEN PART TESTS” Appendix 3: Seven Part Tests Swamp Sclerophyll Forest Swamp Sclerophyll Forest on Coastal Floodplains of the NSW North Coast, Sydney Basin and South East Corner bioregions is listed as an Endangered Ecological Community under the NSW Threatened Species Conservation Act (1995). It is not listed under the schedules of the Commonwealth Environmental Protection and Biodiversity Conservation Act (1999). Swamp Sclerophyll Forest on Coastal Floodplains of the NSW North Coast, Sydney Basin and South East Corner bioregions includes and replaces Sydney Coastal Estuary Swamp Forest in the Sydney Basin bioregion Endangered Ecological Community. This community is associated with humic clay loams and sandy loams, on waterlogged or periodically inundated alluvial flats and drainage lines associated with coastal floodplains (NSW Scientific Committee 2011). It occurs typically as open forests to woodlands, although partial clearing may have reduced the canopy to scattered trees or scrub. The understorey may contain areas of fernland and tall reedland or sedgeland which in turn may also form mosaics with other floodplain communities and often fringe wetlands with semi-permanent standing water (NSW Scientific Committee 2011). Swamp Sclerophyll Forest on Coastal Floodplains generally occurs below 20 metres ASL, often on small floodplains or where the larger floodplains adjoin lithic substrates or coastal sand plains (NSW Scientific Committee 2011). The species composition of Swamp Sclerophyll Forest is primarily determined by the frequency and duration of waterlogging and the texture, salinity nutrient and moisture content of the soil. The species composition of the trees varies considerably, but the most widespread and abundant dominant trees include Eucalyptus robusta Swamp Mahogany, Melaleuca quinquenervia and, south from Sydney, Eucalyptus botryoides Bangalay and Eucalyptus longifolia Woollybutt (OEH 2015a).