Therapeutic Potential of Zingiberaceae in Alzheimer's Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amomum Compactum) and True Cardamom (Elettaria Cardamomum
NUSANTARA BIOSCIENCE ISSN: 2087-3948 Vol. 6, No. 1, pp.94-101 E-ISSN: 2087-3956 May 2014 DOI: 10.13057/nusbiosci/n060115 Short Communication:Comparisons of isozyme diversity in local Java cardamom (Amomum compactum) and true cardamom (Elettaria cardamomum) AHMAD DWI SETYAWAN1,♥, WIRYANTO1, SURANTO1, NURLIANI BERMAWIE2, SUDARMONO3 1Department of Biology, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Jl. Ir. Sutami 36a, Surakarta 57126, Central Java, Indonesia. Tel./fax.: +62-271-663375,♥email: [email protected] 2Indonesian Medicinal and Aromatic Crops Research Institute, Jl. Tentara Pelajar No.3, Cimanggu, Bogor 16111, West Java, Indonesia. 3Bogor Botanic Garden, Indonesian Institute of Sciences, Jl. Ir. H. Juanda No.13, Bogor16122, West Java, Indonesia. Manuscript received: 13 February 2014. Revision accepted: 28 April 2014. Abstract. Setyawan AD, Wiryanto, Suranto, Bermawie N, Sudarmono. 2014. Comparisons of isozyme diversity in local Java cardamom (Amomum compactum) and true cardamom (Elettaria cardamomum). Nusantara Bioscience 6: 94-101. Fruits of Java cardamoms (Amomum compactum) and true cardamoms (Elettaria cardamomum) had long been used as spices, flavoring agent, garnishing plants, etc. This research was conducted to find out: (i) variation of isozymic bands in some population of Java cardamoms and true cardamoms; and (ii) phylogenetic relationship of these cardamoms based on variation of isozymic bands. Plant material (i.e., rhizome) of Java cardamoms was collected from Bogor Botanical Garden, and plant material of true cardamoms was gathered from Indonesian Medicinal and Aromatic Crops Research Institute, Bogor, Indonesia. Ten accessions were assayed in every population. The two isozymic systems were assayed, namely esterase (EST) and peroxidase (PER, PRX). -

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -
Thesis Final Edition
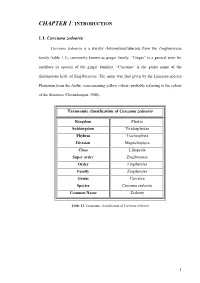
CHAPTER 1: INTRODUCTION 1.1. Curcuma zedoaria Curcuma zedoaria is a starchy rhizomatous/tuberous from the Zingiberaceae family (table 1.1), commonly known as ginger family. “Ginger” is a general term for members or species of the ginger families. “Curcuma” is the genus name of the rhizomatous herb, of Zingiberaceae. The name was first given by the Linnaeus species Plantarum from the Arabic term meaning yellow colour, probably referring to the colour of the rhizomes (Govindarajan, 1980). Taxonomic classification of Curcuma zedoaria Kingdom Plantae Subkingdom Viridaeplantae Phylum Tracheophyta Division Magnoliophyta Class Liliopsida Super order Zingiberanae Order Zingiberales Family Zingiberales Genus Curcuma Curcuma zedoaria Species Common Name Zedoary Table 1.1 Taxonomic classification of Curcuma zedoaria 1 Chapter 1 1.1.1. Description and distribution Curcuma zedoaria is locally known as “kunyit putih” or “temu putih”. It is able to grow up to one and half meters or even more. The leaves are around eighty centimetres long and they usually have a purple-brown flush along the midrib on both surfaces of the leaf. The rhizomes are frequently confused with those of Curcuma aeruginosa because both are of a similar colour (yellow). However, they can be distinguished easily by conducting a cross section on the rhizomes of the mature plants of Curcuma aeruginosa which are slightly dark purplish. In comparison, the colour of the rhizomes of Curcuma zedoaria is pale yellow or white. The rhizomes of Curcuma aeruginosa are highly aromatic due to the high amount of 1, 8-cineol as 25.20% (Ibrahim et al. 2003). Curcuma zedoaria grows mainly in the East-Asian countries including China (called Er- chu in Chinese), Vietnam, India, Bangladesh, Indonesia, Malaysia (can be found at Kuala Selangor, Teluk Intan; Perak, Labis; Johor, and Pahang) and Japan (Islam et al. -

Chemical Composition and Product Quality Control of Turmeric
Stephen F. Austin State University SFA ScholarWorks Faculty Publications Agriculture 2011 Chemical composition and product quality control of turmeric (Curcuma longa L.) Shiyou Li Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Wei Yuan Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Guangrui Deng Ping Wang Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Peiying Yang See next page for additional authors Follow this and additional works at: http://scholarworks.sfasu.edu/agriculture_facultypubs Part of the Natural Products Chemistry and Pharmacognosy Commons, and the Pharmaceutical Preparations Commons Tell us how this article helped you. Recommended Citation Li, Shiyou; Yuan, Wei; Deng, Guangrui; Wang, Ping; Yang, Peiying; and Aggarwal, Bharat, "Chemical composition and product quality control of turmeric (Curcuma longa L.)" (2011). Faculty Publications. Paper 1. http://scholarworks.sfasu.edu/agriculture_facultypubs/1 This Article is brought to you for free and open access by the Agriculture at SFA ScholarWorks. It has been accepted for inclusion in Faculty Publications by an authorized administrator of SFA ScholarWorks. For more information, please contact [email protected]. Authors Shiyou Li, Wei Yuan, Guangrui Deng, Ping Wang, Peiying Yang, and Bharat Aggarwal This article is available at SFA ScholarWorks: http://scholarworks.sfasu.edu/agriculture_facultypubs/1 28 Pharmaceutical Crops, 2011, 2, 28-54 Open Access Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.) ,1 1 1 1 2 3 Shiyou Li* , Wei Yuan , Guangrui Deng , Ping Wang , Peiying Yang and Bharat B. Aggarwal 1National Center for Pharmaceutical Crops, Arthur Temple College of Forestry and Agriculture, Stephen F. -

Well-Known Plants in Each Angiosperm Order
Well-known plants in each angiosperm order This list is generally from least evolved (most ancient) to most evolved (most modern). (I’m not sure if this applies for Eudicots; I’m listing them in the same order as APG II.) The first few plants are mostly primitive pond and aquarium plants. Next is Illicium (anise tree) from Austrobaileyales, then the magnoliids (Canellales thru Piperales), then monocots (Acorales through Zingiberales), and finally eudicots (Buxales through Dipsacales). The plants before the eudicots in this list are considered basal angiosperms. This list focuses only on angiosperms and does not look at earlier plants such as mosses, ferns, and conifers. Basal angiosperms – mostly aquatic plants Unplaced in order, placed in Amborellaceae family • Amborella trichopoda – one of the most ancient flowering plants Unplaced in order, placed in Nymphaeaceae family • Water lily • Cabomba (fanwort) • Brasenia (watershield) Ceratophyllales • Hornwort Austrobaileyales • Illicium (anise tree, star anise) Basal angiosperms - magnoliids Canellales • Drimys (winter's bark) • Tasmanian pepper Laurales • Bay laurel • Cinnamon • Avocado • Sassafras • Camphor tree • Calycanthus (sweetshrub, spicebush) • Lindera (spicebush, Benjamin bush) Magnoliales • Custard-apple • Pawpaw • guanábana (soursop) • Sugar-apple or sweetsop • Cherimoya • Magnolia • Tuliptree • Michelia • Nutmeg • Clove Piperales • Black pepper • Kava • Lizard’s tail • Aristolochia (birthwort, pipevine, Dutchman's pipe) • Asarum (wild ginger) Basal angiosperms - monocots Acorales -

Pharmacological Importance of Kaempferia Galanga (Zingiberaceae): a Mini Review
International Journal of Research in Pharmacy and Pharmaceutical Sciences International Journal of Research in Pharmacy and Pharmaceutical Sciences ISSN: 2455-698X Impact Factor: RJIF 5.22 www.pharmacyjournal.in Volume 3; Issue 3; May 2018; Page No. 32-39 Pharmacological importance of Kaempferia galanga (Zingiberaceae): A mini review Hosne Jahan Shetu1, Kaniz Taskina Trisha2, Shishir Ahmed Sikta3, Raihanatul Anwar4, Sadman Sakib Bin Rashed5, Pritesh Ranjan Dash6* 1, 2, 3 Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh 4, 5 Department of Pharmacy, BRAC University, Mohakhali, Dhaka, Bangladesh 6 Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh Abstract Kaempferia galanga L. belonging to the family Zingiberaceae is an endangered medicinal plant with potent medicinal activities. The leaves, rhizome and root tubers of the plant possess a number of medicinal applications. The plant is economically important and is over exploited to the extent that there is always scarcity of propagating material (rhizomes) which is the consumable part too. The present review provides broad information of Kaempferia galanga throwing light on its current status, ethnobotany, phytochemistry and pharmacology. Extracts of Kaempferia galanga have anti-inflammatory, analgesic, anti-diarrheal, anti- bacterial, sedative, cytotoxic, insecticidal and anthelmintic properties which are reported here. Keywords: Kaempferia galanga, zingiberaceae, phytochemistry, pharmacological activity Introduction spite of the variety of useful pharmacological properties it Kaempferia galanga Linn., commonly known as Cekor, possess. Therefore, the importance of the plant K. galanga as Ekangi, Kencur or aromatic ginger is a stem less herb in a medicinal plant is to be documented and presented to the Zingiberaceae family. The plant is native to tropical Asia mass of people. -

– the 2020 Horticulture Guide –
– THE 2020 HORTICULTURE GUIDE – THE 2020 BULB & PLANT MART IS BEING HELD ONLINE ONLY AT WWW.GCHOUSTON.ORG THE DEADLINE FOR ORDERING YOUR FAVORITE BULBS AND SELECTED PLANTS IS OCTOBER 5, 2020 PICK UP YOUR ORDER OCTOBER 16-17 AT SILVER STREET STUDIOS AT SAWYER YARDS, 2000 EDWARDS STREET FRIDAY, OCTOBER 16, 2020 SATURDAY, OCTOBER 17, 2020 9:00am - 5:00pm 9:00am - 2:00pm The 2020 Horticulture Guide was generously underwritten by DEAR FELLOW GARDENERS, I am excited to welcome you to The Garden Club of Houston’s 78th Annual Bulb and Plant Mart. Although this year has thrown many obstacles our way, we feel that the “show must go on.” In response to the COVID-19 situation, this year will look a little different. For the safety of our members and our customers, this year will be an online pre-order only sale. Our mission stays the same: to support our community’s green spaces, and to educate our community in the areas of gardening, horticulture, conservation, and related topics. GCH members serve as volunteers, and our profits from the Bulb Mart are given back to WELCOME the community in support of our mission. In the last fifteen years, we have given back over $3.5 million in grants to the community! The Garden Club of Houston’s first Plant Sale was held in 1942, on the steps of The Museum of Fine Arts, Houston, with plants dug from members’ gardens. Plants propagated from our own members’ yards will be available again this year as well as plants and bulbs sourced from near and far that are unique, interesting, and well suited for area gardens. -

Micropropagation-An in Vitro Technique for the Conservation of Alpinia Galanga
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2014, 5(3):259-263 ISSN: 0976-8610 CODEN (USA): AASRFC Micropropagation-an in vitro technique for the conservation of Alpinia galanga Nongmaithem M. Singh 1, Lukram A. Chanu 1, Yendrembam P. Devi 1, Wahengbam R.C. Singh 2 and Heigrujam B. Singh 2 1DBT-Institutional Biotech Hub, Pettigrew College, Ukhrul, Manipur 2DBT- Institutional Biotech Hub, Deptt. of Biotechnology, S.K. Women’s College, Nambol, Manipur _____________________________________________________________________________________________ ABSTRACT This study was conducted to develop an efficient protocol for mass propagation of Alpinia galanga L. Explants from rhizome buds were cultured on Murashige and Skoog (MS) medium supplemented with 6-Benzylaminopurine (BAP) alone (0 to 5 mg/l) or a combination of BAP (0 to 5 mg/l) and indole 3-acetic acid (IAA) (0 to 2 mg/l). MS medium supplemented with a combination of 5.0 mg/l BAP and 2.0 mg/l IAA or 3.0 mg/l BAP and 0.5 mg/l IAA produced the highest mean number of shoots per explant as compared to other concentrations. The best shoot length was obtained on the medium containing 1.0 mg/l of BAP and 2.0 mg/l IAA. Thus, combined effects of BAP and IAA improved significantly the shoot growth and proliferation. MS medium supplemented with a combination of 5.0 mg/l BAP and 2 mg/l IAA gave the highest number of roots. However, longest roots per explant were obtained with 1.0 mg/l BAP alone. -

The Molecular Phylogeny of Alpinia (Zingiberaceae): a Complex and Polyphyletic Genus of Gingers1
American Journal of Botany 92(1): 167±178. 2005. THE MOLECULAR PHYLOGENY OF ALPINIA (ZINGIBERACEAE): A COMPLEX AND POLYPHYLETIC GENUS OF GINGERS1 W. J OHN KRESS,2,3,5 AI-ZHONG LIU,2 MARK NEWMAN,4 AND QING-JUN LI3 2Department of Botany, MRC-166, United States National Herbarium, National Museum of Natural History, Smithsonian Institution, PO Box 37012, Washington, D.C. 20013-7012 USA; 3Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan 666303 China; and 4Royal Botanic Garden, 20A Inverleith Row, Edinburgh EH3 5LR, Scotland, UK Alpinia is the largest, most widespread, and most taxonomically complex genus in the Zingiberaceae with 230 species occurring throughout tropical and subtropical Asia. Species of Alpinia often predominate in the understory of forests, while others are important ornamentals and medicinals. Investigations of the evolutionary relationships of a subset of species of Alpinia using DNA sequence- based methods speci®cally test the monophyly of the genus and the validity of the previous classi®cations. Seventy-two species of Alpinia, 27 non-Alpinia species in the subfamily Alpinioideae, eight species in the subfamily Zingiberoideae, one species in the subfamily Tamijioideae, and three species in the outgroup genus Siphonochilus (Siphonochiloideae) were sequenced for the plastid matK region and the nuclear internal transcribed spacer (ITS) loci. Parsimony analyses of both individual and combined data sets identi®ed six polyphyletic clades containing species of Alpinia distributed across the tribe Alpinieae. These results were supported by a Bayesian analysis of the combined data set. Except in a few speci®c cases, these monophyletic groupings of species do not correspond with either Schumann's (1904) or Smith's (1990) classi®cation of the genus. -

C-23 Phytochemical of Kaempferia Plant And
Proceeding of International Conference On Research, Implementation And Education Of Mathematics And Sciences 2014, Yogyakarta State University, 18-20 May 2014 C-23 PHYTOCHEMICAL OF KAEMPFERIA PLANT AND BIOPROSPECTING FOR CANCER TREATMENT Sri Atun Chemistry education Faculty of Mathematical and Natural Science, Yogyakarta State University, Jl. Colombo No. 1 Yogyakarta, Indonesia, 55281 e-mail : [email protected] ABSTRACT Kaempferia genus is perennial member of the Zingiberaceae family and is cultivated in Indonesia and other parts of Southeast Asia. Number of studies has been conducted, providing information related to Kaempferia as antioxidant; antimutagenic; and chemopreventive agent. This paper reports some isolated compounds from this plant, biological activity, and bioprospecting for cancer treatment. Keyword: Cancer treatment; Kaempferia; Zingiberaceae INTRODUCTION Kaempferia is a genus, belong to family of Zingiberaceae. This plant grows in Southeast Asia, India, Sri Lanka, Indonesia, and Southem China. Kaempferia genus sinonim with Boesenbergia genus by Baker. This plant has 8 different botanical names which are Boesenbergia cochinchinensis (Gagnep.) Loes., Boesenbergia pandurata (Roxb.) Schltr., Curcuma rotunda L., Gastrochilus panduratus (Roxb.) Ridl., Gastrochilus rotundus (L.) Alston, Kaempferia cochinchinensis Gagnep., Kaempferia ovate Roscoe, Kaempferia galanga, Kaempferia rotunda, and Kaempferia pandurata Roxb nonetheless it is currently known as Boesenbergia rotunda (L.)Mansf (Tan Eng-Chong, et. al, 2012). The plants grown naturally in damp, shaded parts of the lowland or on hill slopes, as scattered plants or thickets. Economically important species among the plant families, the Zingiberaceae, which are perennial rhizomatous herbs, contain volatile oil and other important compounds of enormous medicinal values (Singh C.B., 2013). Phytochemical and biologycal activities of some species of Kaempferia Phytochemical and biologycal some species of plants of the genus Kaempferia reported by many researchers, among others: 1. -

2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Check List of Wild Angiosperms of Bhagwan Mahavir (Molem
Check List 9(2): 186–207, 2013 © 2013 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Check List of Wild Angiosperms of Bhagwan Mahavir PECIES S OF Mandar Nilkanth Datar 1* and P. Lakshminarasimhan 2 ISTS L (Molem) National Park, Goa, India *1 CorrespondingAgharkar Research author Institute, E-mail: G. [email protected] G. Agarkar Road, Pune - 411 004. Maharashtra, India. 2 Central National Herbarium, Botanical Survey of India, P. O. Botanic Garden, Howrah - 711 103. West Bengal, India. Abstract: Bhagwan Mahavir (Molem) National Park, the only National park in Goa, was evaluated for it’s diversity of Angiosperms. A total number of 721 wild species belonging to 119 families were documented from this protected area of which 126 are endemics. A checklist of these species is provided here. Introduction in the National Park are Laterite and Deccan trap Basalt Protected areas are most important in many ways for (Naik, 1995). Soil in most places of the National Park area conservation of biodiversity. Worldwide there are 102,102 is laterite of high and low level type formed by natural Protected Areas covering 18.8 million km2 metamorphosis and degradation of undulation rocks. network of 660 Protected Areas including 99 National Minerals like bauxite, iron and manganese are obtained Parks, 514 Wildlife Sanctuaries, 43 Conservation. India Reserves has a from these soils. The general climate of the area is tropical and 4 Community Reserves covering a total of 158,373 km2 with high percentage of humidity throughout the year.