Computational Analysis of Coronary Blood Flow: the Role of Asynchronous Pacing and Arrhythmias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physiology of Heart Unit-4 (ZOOA-CC4-9-TH)
Physiology of Heart Unit-4 (ZOOA-CC4-9-TH) Coronary Circulation: The heart muscle, like every other organ or tissue in your body, needs oxygen-rich blood to survive. Blood is supplied to the heart by its own vascular system, called coronary circulation. The aorta (the main blood supplier to the body) branches off into two main coronary blood vessels (also called arteries). These coronary arteries branch off into smaller arteries, which supply oxygen-rich blood to the entire heart muscle. The right coronary artery supplies blood mainly to the right side of the heart. The right side of the heart is smaller because it pumps blood only to the lungs. The left coronary artery, which branches into the left anterior descending artery and the circumflex artery, supplies blood to the left side of the heart. The left side of the heart is larger and more muscular because it pumps blood to the rest of the body. Coronary circulation is the circulation of blood in the blood vessels that supply the heart muscle (myocardium). Coronary arteries supply oxygenated blood to the heart muscle, and cardiac veins drain away the blood once it has been deoxygenated. Because the rest of the body, and most especially the brain, needs a steady supply of oxygenated blood that is free of all but the slightest interruptions, the heart is required to function continuously. Therefore its circulation is of major importance not only to its own tissues but to the entire body and even the level of consciousness of the brain from moment to moment. -

Abnormally Enlarged Singular Thebesian Vein in Right Atrium
Open Access Case Report DOI: 10.7759/cureus.16300 Abnormally Enlarged Singular Thebesian Vein in Right Atrium Dilip Kumar 1 , Amit Malviya 2 , Bishwajeet Saikia 3 , Bhupen Barman 4 , Anunay Gupta 5 1. Cardiology, Medica Institute of Cardiac Sciences, Kolkata, IND 2. Cardiology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 3. Anatomy, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 4. Internal Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 5. Cardiology, Vardhman Mahavir Medical College (VMMC) and Safdarjung Hospital, New Delhi, IND Corresponding author: Amit Malviya, [email protected] Abstract Thebesian veins in the heart are subendocardial venoluminal channels and are usually less than 0.5 mm in diameter. The system of TV either opens a venous (venoluminal) or an arterial (arterioluminal) channel directly into the lumen of the cardiac chambers or via some intervening spaces (venosinusoidal/ arteriosinusoidal) termed as sinusoids. Enlarged thebesian veins are reported in patients with congenital heart disease and usually, multiple veins are enlarged. Very few reports of such abnormal enlargement are there in the absence of congenital heart disease, but in all such cases, they are multiple and in association with coronary artery microfistule. We report a very rare case of a singular thebesian vein in the right atrium, which was abnormally enlarged. It is important to recognize because it can be confused with other cardiac structures like coronary sinus during diagnostic or therapeutic catheterization and can lead to cardiac injury and complications if it is attempted to cannulate it or pass the guidewires. -

REVIEW ARTICLE Anatomy and Physiology of Coronary Blood Flow
REVIEW ARTICLE Anatomy and physiology of coronary blood flow Heinrich R. Schelbert, MD, PhD INTRODUCTION hypertrophic cardiomyopathy, or coronary artery disease, resting myocardial blood flows frequently are similar to Regional myocardial blood flow can now be mea- those in normal individuals.17-19 It is the response of sured noninvasively in units of milliliters blood per myocardial blood flow to specifically-targeted pharma- minute per gram myocardium. These noninvasive mea- cological or physiological interventions that can uncover surements are not confined to a specific imaging the presence of functional or structural disease-related modality but are available with MRI, CT, and PET, alterations of the coronary circulation. This then under- although, thus far, most investigations of the coronary scores the need for closely examining these targets and circulation in humans have employed PET flow mea- how they relate to anatomical and functional determi- surements. Flow estimates with these different imaging nants of coronary blood flow and, by inference, to modalities were found in animal experiments to correlate myocardial blood flow and their alterations in cardio- well with invasive flow estimates by the arterial blood vascular disease. Local and systemic mechanisms sampling-microsphere technique widely considered as regulate the complex interactions between flow and the ‘‘gold standard’’ of flow measurements.1-11 In these anatomy in order to meet the heart’s energy needs. comparison studies, noninvasively-derived estimates A comprehensive description of the coronary circulatory corresponded linearly with invasively-measured myo- function and its control exceeds the scope of this review cardial blood flows over a wide flow range, i.e., from as so that the interested reader is referred to detailed reviews low as 0.3 mL/minute/g to as high as 5-6 mL/minute/g. -

22. Heart.Pdf
CARDIOVASCULAR SYSTEM OUTLINE 22.1 Overview of the Cardiovascular System 657 22.1a Pulmonary and Systemic Circulations 657 22.1b Position of the Heart 658 22 22.1c Characteristics of the Pericardium 659 22.2 Anatomy of the Heart 660 22.2a Heart Wall Structure 660 22.2b External Heart Anatomy 660 Heart 22.2c Internal Heart Anatomy: Chambers and Valves 660 22.3 Coronary Circulation 666 22.4 How the Heart Beats: Electrical Properties of Cardiac Tissue 668 22.4a Characteristics of Cardiac Muscle Tissue 668 22.4b Contraction of Heart Muscle 669 22.4c The Heart’s Conducting System 670 22.5 Innervation of the Heart 672 22.6 Tying It All Together: The Cardiac Cycle 673 22.6a Steps in the Cardiac Cycle 673 22.6b Summary of Blood Flow During the Cardiac Cycle 673 22.7 Aging and the Heart 677 22.8 Development of the Heart 677 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch22_656-682.indd 656 2/14/11 4:29 PM Chapter Twenty-Two Heart 657 n chapter 21, we discovered the importance of blood and the which carry blood back to the heart. The differences between I myriad of substances it carries. To maintain homeostasis, blood these types of vessels are discussed in chapter 23. Most arteries must circulate continuously throughout the body. The continual carry blood high in oxygen (except for the pulmonary arteries, pumping action of the heart is essential for maintaining blood as explained later), while most veins carry blood low in oxygen circulation. If the heart fails to pump adequate volumes of blood, (except for the pulmonary veins). -
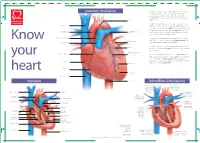
Structure Coronary Circulation
B C M Y X CMY B5 C5 M5 Y5 X5 B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y Prinect/FOGRA 5 Dipco 2.1 Format 105 © 2004 FOGRA/Heidelberger Druckmaschinen AG B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y X B C M Y X B C M Y X B5 C5 M5 Y5 X5 CMY B C M Y X B C M Y X CMY B5 C5 M5 Y5 X5 B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y Prinect/FOGRA 5 Dipco 2.1 Format 105 © 2004 FOGRA/Heidelberger Druckmaschinen AG B C M Y X 40% 80% B C M Y X B C M Y X 40% 80% B C M Y X B C M Y X B C M Y X B5 C5 M5 Y5 X5 CMY B C M Y X 5 5 5 5 5 5 5 5 5 5 4 4 4 4 4 4 4 4 4 4 3 3 3 3 3 3 3 3 3 3 2 2 2 2 2 2 2 2 2 2 1 1 1 1 1 1 1 1 1 1 1234567891011121314151617181920212223242526272829303132 coronary circulation The heart is a continuous pump which distributes blood to every part of the body. Blood carries essential oxygen to the tissues and carries away unwanted carbon dioxide and other waste products. -

Structure of the Coronary Circulation
CORONARY PHYSIOLOGY IN THE CATHLAB: STRUCTURE OF THE CORONARY CIRCULATION Educational Training Program ESC European Heart House april 24th - 26th 2014 Nico H. J. Pijls, MD, PhD Catharina Hospital, Eindhoven, The Netherlands Disclosures related to this ETP course: • Dr Pijls received institutional research grants from St Jude Medical and Pharma Solutions • Dr Pijls is consultant to St Jude Medical, and to Heartflow ISSUES TO BE DISCUSSED • structure of the coronary circulation • relation between vessel size and perfusion area • endothelium and development of atherosclerosis • the 2 or 3 compartment model of the coron circulation • collaterals ( to be discussed tomorrow) • 03 cc/schema Braunwald 03 cc/schema Braunwald Let’s have a closer look at the coronary tree….. Fractale structure of the coronary circulation ( Gould, Finet) X 1 X 0.75 X 4.5 X 10.5 X 90 X 1100 epicardial microvascular compartment compartment ( > 400 µm) traditionally visible by angiography and more recently by many invasive Black box and non-invasive imaging methods (until recently) Regulation of coronary blood flow by arteriolar sphincters To be further discussed by Dirk Duncker ISSUES TO BE DISCUSSED • structure of the coronary circulation • relation between vessel size and perfusion area • endothelium and development of atherosclerosis • the 2 or 3 compartment model of the coron circulation • collaterals Relationship between vessel size and myocardial mass Cross Sectional Area (~ flow) Vessel Diameter (mm) 6 5 4 3 2 1 0 0 100 200 300 400 500 Regional Myocardial Mass -

Lab 3 Heart Sounds, Valve Problems and Blood Flow
Lab 3 Heart Sounds, Valve Problems and Blood Flow MDufilho 1 Heart Sound Lub-dup, lub-dup, lub-dup • Lub – lower pitch • Dup – higher pitch • Normal heart sounds -Animated Normal S1 S2 MDufilho 2 Figure 18.20 Areas of the thoracic surface where the sounds of individual valves can best be detected. Aortic valve sounds heard in 2nd intercostal space at right sternal margin Pulmonary valve sounds heard in 2nd intercostal space at left sternal margin Mitral valve sounds heard over heart apex (in 5th intercostal space) in line with middle of clavicle Tricuspid valve sounds typically heard in right sternal margin of 5th MDufilho intercostal space 3 Figure 18.5e – Heart Valves Aorta Left pulmonary artery Superior vena cava Right pulmonary artery Left atrium Left pulmonary veins Pulmonary trunk Right atrium Mitral (bicuspid) valve Right pulmonary veins Fossa ovalis Aortic valve Pectinate muscles Pulmonary valve Tricuspid valve Right ventricle Left ventricle Chordae tendineae Papillary muscle Interventricular septum Trabeculae carneae Epicardium Inferior vena cava Myocardium Endocardium Frontal section MDufilho 4 (not in text) Valve Prolapse MDufilho 5 (not in text) Valve Prolapse MDufilho 6 (not in text) Valvular Stenosis MDufilho 7 (not in text) Valvular Stenosis Heart Sounds S3 S4 Murmurs MDufilho 8 Figure 18.5b Blood Supply to the Myocardium Left common carotid artery Brachiocephalic trunk Left subclavian artery Superior vena cava Aortic arch Ligamentum arteriosum Right pulmonary artery Left pulmonary artery Ascending aorta Left pulmonary -

CARDIOLOGY PHYSIOLOGY Part 1 Describe the Adult Coronary Circulation the Left Main and Right Coronary Arteries Originate At
CARDIOLOGY PHYSIOLOGY Part 1 Describe the adult coronary circulation The left main and right coronary arteries originate at the base of the aorta, from the coronary ostia, which is located behind the aortic leaflets. These two arteries lie on the surface of the heart whereas the smaller arteries penetrate the epicardium and supply blood to the myocardium. The left main coronary artery arises from the posterior aortic sinus above the left coronary cusp of the aortic valve and has two main branches, the circumflex and the left anterior descending artery. The circumflex artery lies in the left atrioventricular groove and supplies most of the left atrium and the posterior and lateral parts of the left ventricle. It, also with the left anterior descending artery, supplies the anterolateral papillary muscle. The circumflex artery has several branches including; atrial branches and obtuse marginal branches 1, 2 and 3. In 10% of people the circumflex supplies the posterior descending artery, this is termed ‘left dominant circulation’. Also, in 40% of the population, the circumflex supplies the sinoatrial node. The left anterior descending artery, which is also known as the anterior interventricular branch, follows the anterior interventricular sulcus down to the apex of the heart where it often anastomoses with the terminal branches of the posterior descending artery. It supplies the anterolateral portions of the heart, the anterior 2/3 of the interventricular septum. Important branches include the diagonal branches, which run laterally to supply the anterolateral wall of the LV, and the septal branches which supplies the anterior 2/3 of the interventricular septum. -

Circulatory System WARM-UP
_____ 1. Of the following, which is NOT involved in pulmonary circulation? a. Coronary Artery c. Right Ventricle b. Left Atrium d. Pulmonary Vein a. CORONARY ARTERY _____ 2. The heart of a human contains ______ chamber(s). a. One c. three b. Two d. four d. FOUR _____ 3. Blood is a tissue that consists of ____________. a. Cells c. liquid b. Cell fragments d. all of the above d. all of the above _____ 4. Of the following, which is NOT involved in systemic circulation? a. Aorta c. inferior vena cava b. Superior vena cava d. Pulmonary artery d. Pulmonary artery _____ 5. Of the following, which is NOT a function of blood? a. Digestion c. Carries oxygen b. Carries waste products d. Carries nutrients a. Digestion MATCH THE ANSWER WITH THE GIVEN QUESTION a. Capillaries f. Atria b. Pulmonary circulation g. Coronary circulation c. Arteries h. Ventricles d. Systemic Circulation i. Veins e. Blood Pressure _____ 6. Upper chambers of the heart f. Atria (plural form of Atrium) MATCH THE ANSWER WITH THE GIVEN QUESTION a. Capillaries f. Atria b. Pulmonary circulation g. Coronary circulation c. Arteries h. Ventricles d. Systemic Circulation i. Veins e. Blood Pressure _____ 7. Vessels that move blood toward the heart i. Veins MATCH THE ANSWER WITH THE GIVEN QUESTION a. Capillaries f. Atria b. Pulmonary circulation g. Coronary circulation c. Arteries h. Ventricles d. Systemic Circulation i. Veins e. Blood Pressure _____8. Vessels that move blood away from the heart c. arteries MATCH THE ANSWER WITH THE GIVEN QUESTION a. Capillaries f. -

The Cardiovascular System
Heart’s Place in the Circulation Essentials of Anatomy & Physiology, 4th Edition Martini / Bartholomew Heart Pumps Blood into Two Circuits in Sequence The Cardiovascular 1. Pulmonary circuit • To and from the lungs 12 System: The Heart 2. Systemic circuit • To and from the rest of the body PowerPoint® Lecture Outlines prepared by Alan Magid, Duke University Slides 1 to 65 Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Heart’s Place in the Circulation Heart’s Place in the Circulation Three Kinds of Blood Vessels Two Sets of Pumping Chambers in Heart 1. Arteries 1. Right atrium • Carry blood away from heart and carry it to • Receives systemic blood the capillaries 2. Right ventricle 2. Capillaries • Pumps blood to lungs (pulmonary) • Connect arteries and veins 3. Left atrium • Exchange area between blood and cells • Receives blood from lungs 3. Veins 4. Left ventricle • Receive blood from capillaries and carry it • Pumps blood to organ systems (systemic) back to the heart Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings Heart’s Place in the Circulation The Anatomy of the Heart Overview of the Pericardial Cavity Cardiovascular • Surrounds the heart System • Lined by pericardium • Two layers 1. Visceral pericardium (epicardium) • Covers heart surface 2. Parietal pericardium • Lines pericardial sac that surrounds heart Figure 12-1 Copyright © 2007 Pearson Education, Inc., publishing as Benjamin Cummings 1 The Anatomy of the Heart The Anatomy of the Heart The Location of the Heart in the Thoracic Cavity Surface Features of the Heart 1. -

Pathophysiology of Coronary Circulation'
Br Heart J: first published as 10.1136/hrt.33.2.173 on 1 March 1971. Downloaded from British Heart Journal, I971, 33, I73-I84. Pathophysiology of coronary circulation' Richard S. Ross2 From The Johns Hopkins University, School of Medicine, *Baltimore, Maryland, U.S.A. I consider it a great honour to have been asked like to quote briefly from the introduction to to give the Sir Thomas Lewis Lecture, and I the first Herter Lecture as follows: have chosen for my subject the pathophysi- ,,ology of the coronary circulation - a topic 'Laboratory methods as applied to the which was of great interest to Sir Thomas. study of clinical medicine have come to stay; reason this is instruments and methods of precision are Another for selecting topic that gradually relieving medicine of its past so much of the good, recent work in this field stigma; they are lifting it to the plane of its * has been done in Britain and many of the sister sciences, its true and proper status. investigators who have made significant con- We have been too content in the past with tfributions are members of this distinguished opinion. In the future we shall rest our case society. This may possibly be a disadvantage upon fact.' (Lewis, I9I5.) m that I will have difficulty saying anything W which is not well known to a large segment of I would like to turn to the subject of my the audience. A third reason for this selection lecture by examining the history of clinical is that ischaemic heart disease is of interest to investigation of ischaemic heart disease. -

The Cardiovascular System: the Heart: Part A
PowerPoint® Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College C H A P T E R 18 The Cardiovascular System: The Heart: Part A © Annie Leibovitz/Contact Press Images © 2013 Pearson Education, Inc. The Pulmonary and Systemic Circuits • Heart is transport system; two side-by-side pumps – Right side receives oxygen-poor blood from tissues • Pumps to lungs to get rid of CO2, pick up O2, via pulmonary circuit – Left side receives oxygenated blood from lungs • Pumps to body tissues via systemic circuit © 2013 Pearson Education, Inc. Figure 18.1 The systemic and pulmonary circuits. Capillary beds of lungs where gas exchange occurs Pulmonary Circuit Pulmonary arteries Pulmonary veins Aorta and branches Venae cavae Left atrium Left Right ventricle atrium Heart Right ventricle Systemic Circuit Capillary beds of all body tissues where Oxygen-rich, gas exchange occurs CO2-poor blood Oxygen-poor, CO2-rich blood © 2013 Pearson Education, Inc. Heart Anatomy • Approximately size of fist • Location: – In mediastinum between second rib and fifth intercostal space – On superior surface of diaphragm – Two-thirds of heart to left of midsternal line – Anterior to vertebral column, posterior to sternum PLAY Animation: Rotatable heart © 2013 Pearson Education, Inc. Heart Anatomy • Base (posterior surface) leans toward right shoulder • Apex points toward left hip • Apical impulse palpated between fifth and sixth ribs, just below left nipple © 2013 Pearson Education, Inc. Figure 18.2a Location of the heart in the mediastinum. Midsternal line 2nd rib Sternum Diaphragm Location of apical impulse © 2013 Pearson Education, Inc. Figure 18.2c Location of the heart in the mediastinum.