Salinity Effects on Native and Introduced SAV of Suisun Bay and the Delta 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Desilva Island
SUISUN BAY 139 SUISUN BAY 140 SUISUN BAY SUISUN BAY Located immediately downstream of the confluence of the Sacramento and San Joaquin Rivers, Suisun Bay is the largest contiguous wetland area in the San Francisco Bay region. Suisun Bay is a dynamic, transitional zone between the freshwater input of the Central Valley rivers and the tidal influence of the upper San Francisco Estuary. This area supports a substantial number of nesting herons and egrets, including three of the largest colonies in the region. Although suburban development is rampant along the nearby Interstate 80 corridor to the north, most of the Suisun Bay area is protected from heavy development by the California Department of Fish and Game and a number of private duck clubs. Black- Active Great crowned or year Site Blue Great Snowy Night- Cattle last # Colony Site Heron Egret Egret Heron Egret County active Page 501 Bohannon Solano Active 142 502 Campbell Ranch Solano Active 143 503 Cordelia Road Solano 1998 145 504 Gold Hill Solano Active 146 505 Green Valley Road Solano Active 148 506 Hidden Cove Solano Active 149 507 Joice Island Solano 1994 150 508 Joice Island Annex Solano Active 151 509 Sherman Lake Sacramento Active 152 510 Simmons Island Solano 1994 153 511 Spoonbill Solano Active 154 512 Tree Slough Solano Active 155 513 Volanti Solano Active 156 514 Wheeler Island Solano Active 157 SUISUN BAY 141 142 SUISUN BAY Bohannon Great Blue Herons and Great Egrets nest in a grove of eucalyptus trees on a levee in Cross Slough, about 1.8 km east of Beldons Landing. -

San Francisco Bay Plan
San Francisco Bay Plan San Francisco Bay Conservation and Development Commission In memory of Senator J. Eugene McAteer, a leader in efforts to plan for the conservation of San Francisco Bay and the development of its shoreline. Photo Credits: Michael Bry: Inside front cover, facing Part I, facing Part II Richard Persoff: Facing Part III Rondal Partridge: Facing Part V, Inside back cover Mike Schweizer: Page 34 Port of Oakland: Page 11 Port of San Francisco: Page 68 Commission Staff: Facing Part IV, Page 59 Map Source: Tidal features, salt ponds, and other diked areas, derived from the EcoAtlas Version 1.0bc, 1996, San Francisco Estuary Institute. STATE OF CALIFORNIA GRAY DAVIS, Governor SAN FRANCISCO BAY CONSERVATION AND DEVELOPMENT COMMISSION 50 CALIFORNIA STREET, SUITE 2600 SAN FRANCISCO, CALIFORNIA 94111 PHONE: (415) 352-3600 January 2008 To the Citizens of the San Francisco Bay Region and Friends of San Francisco Bay Everywhere: The San Francisco Bay Plan was completed and adopted by the San Francisco Bay Conservation and Development Commission in 1968 and submitted to the California Legislature and Governor in January 1969. The Bay Plan was prepared by the Commission over a three-year period pursuant to the McAteer-Petris Act of 1965 which established the Commission as a temporary agency to prepare an enforceable plan to guide the future protection and use of San Francisco Bay and its shoreline. In 1969, the Legislature acted upon the Commission’s recommendations in the Bay Plan and revised the McAteer-Petris Act by designating the Commission as the agency responsible for maintaining and carrying out the provisions of the Act and the Bay Plan for the protection of the Bay and its great natural resources and the development of the Bay and shore- line to their highest potential with a minimum of Bay fill. -

Northern San Francisco Bay Ecological Risk Assessment: Potential Crude by Rail Incident Meagan Bowis University of San Francisco, [email protected]
The University of San Francisco USF Scholarship: a digital repository @ Gleeson Library | Geschke Center Master's Projects and Capstones Theses, Dissertations, Capstones and Projects Spring 5-20-2016 Northern San Francisco Bay Ecological Risk Assessment: Potential Crude by Rail Incident Meagan Bowis University of San Francisco, [email protected] Follow this and additional works at: https://repository.usfca.edu/capstone Part of the Environmental Health and Protection Commons, Environmental Indicators and Impact Assessment Commons, Natural Resource Economics Commons, Natural Resources Management and Policy Commons, Oil, Gas, and Energy Commons, and the Other Oceanography and Atmospheric Sciences and Meteorology Commons Recommended Citation Bowis, Meagan, "Northern San Francisco Bay Ecological Risk Assessment: Potential Crude by Rail Incident" (2016). Master's Projects and Capstones. 340. https://repository.usfca.edu/capstone/340 This Project/Capstone is brought to you for free and open access by the Theses, Dissertations, Capstones and Projects at USF Scholarship: a digital repository @ Gleeson Library | Geschke Center. It has been accepted for inclusion in Master's Projects and Capstones by an authorized administrator of USF Scholarship: a digital repository @ Gleeson Library | Geschke Center. For more information, please contact [email protected]. This Master’s Project Northern San Francisco Bay Ecological Risk Assessment: Potential Crude by Rail Incident By Meagan Kane Bowis is submitted in partial fulfillment of the requirements -

F-1'7-03 Meeting
MINUTE ITEM This Calendar Item No. <!I/ was approved as Minute Item No . .J.L. by the California State Lands Commission by a vote of _g__ to,L'at Its f-1'7-03 meeting. CALENDAR ITEM C11 A) 11 08/19/03 PRC 5438 WP 5438.1 S) 2 L. Burks AMENDMENT OF MASTER LEASE NO. PRC 5438.1 (ADDENDUM NO. 14) APPLICANT: Pacific Gas and Electric Company AREA, LAND TYPE, AND LOCATION: Master Lease: Over 100 waterway crossings throughout the State. Amendment: Delete from the Lease 6.07 acres, more or less, of tide and submerged lands in Honker and Suisun Bays, near Bay Point, Contra Costa and Solano counties. AUTHORIZED USE: Master Lease: Continued use and maintenance of distribution pipelines to transport natural and synthetic gas. Amendment: Removal of an existing 10-inch and 12-inch diameter pipeline known as PG&E Line 182. LEASE TERM: Existing Master Lease: 20 years, beginning January 1, 1978. A new lease is currently being negotiated. The existing lease is in holdover. CONSIDERATION: Existing Master Lease: $30,400 per year; with the State reserving the right to fix a different rent periodically during the lease term, as provided in the lease. SPECIFIC LEASE PROVISIONS: Liability insurance: $10,000,000 per occurrence for bodily injury and $10,000,000 for property damage. n1 r 01. I lJs l 1U·"t REVISED 08/18/03 0 -1- CALEMDAR PAGE Mir<UT E PA GE CALENDAR ITEM NO. C11 (CONT'D) PROPOSED AMENDMENT: The Master Lease provides that the lease may periodically be amended by a series of addenda for the purpose of adding to and deleting from the lease the parcels of land necessary for the distribution of natural gas pipelines. -

Contents and Acknowledgements
ANNOTATED ATLAS AND IMPLICATIONS FOR THE CONSERVATION OF HERON AND EGRET NESTING COLONIES IN THE SAN FRANCISCO BAY AREA JOHN P. KELLY, KATIE ETIENNE, CHERYL STRONG, MARK MCCAUSTLAND, AND MICHAEL L. PARKES ACR Technical Report 90-3-17 © August 2006, Audubon Canyon Ranch P. O. Box 808, Marshall, CA 94940 © Heron and egret photographs by Philip Loring Greene (http://www.philiplgreene.com) Audubon Canyon Ranch was founded in 1962 to protect an important heronry on the Marin County coast north of San Francisco and to prevent intensive commercial development of the undisturbed area surrounding the colony. Now, Audubon Canyon Ranch manages a system of wildlife sanctuaries in Marin and Sonoma counties and conducts regional programs in conservation research, habitat protection and restoration, and nature education. The San Francisco Bay Bird Observatory (http://www.sfbbo.org), a collaborator on this project, is dedicated to the conservation of birds and their habitats through science and outreach, and to contributing to informed resource management decisions in the San Francisco Bay Area. An electronic version of this document can be downloaded from the Audubon Canyon Ranch website at http://www.egret.org. ACKNOWLEDGEMENTS We thank the following field observers for their assistance in monitoring San Francisco Bay area heronries during 1991-2005: Debbie Ablin, Nancy Abreu, Dawn Adams, Nancy Adess, Russ Agnew, Shirle Akers, Joy Albertson, Catherine Alexander, Bob Anderson, Brenda Anderson, Janica Anderson, Jennie Anderson, Jim Anderson, Andy Angel, -

Montezuma II Wind Energy Project Final Environmental Impact Report
Montezuma II Wind Energy Project Final Environmental Impact Report Prepared for Solano County Department of Resource Management Prepared by Point Impact Analysis, LLC 2555 Park Boulevard, Suite 10 Palo Alto, CA 94306 Telephone: 650.856.2800 Fax: 650.856.2801 Email: [email protected] June 20, 2011 This page intentionally left blank Montezuma II Wind Energy Project Final Environmental Impact Report Prepared for Solano County Department of Resource Management Prepared by Point Impact Analysis, LLC 2555 Park Boulevard, Suite 10 Palo Alto, CA 94306 Telephone: 650.856.2800 Fax: 650.856.2801 Email: srussell@pointim pactanal ysis.com June 20, 2011 This page intentionally left blank TABLE OF CONTENTS This page intentionally left blank Table of Contents MONTEZUMA II WIND ENERGY PROJECT FINAL EIR TABLE OF CONTENTS VOLUME I Chapter Page 1 INTRODUCTION ......................................................................................................................1-1 1.1 Changes Since the Draft EIR ...................................................................................................................... 1-2 1.2 Organization of the Final EIR..................................................................................................................... 1-3 2 ADDITIONAL INFORMATION ............................................................................................. 2-1 2.1 Additional Project Information .................................................................................................................. -

San Francisco Estuary Invasive Spartina Project 2015-16 Isp Monitoring and Treatment Report
2015-16 SAN FRANCISCO ESTUARY INVASIVE SPARTINA PROJECT 2015-16 ISP MONITORING AND TREATMENT REPORT San Francisco Estuary Invasive Spartina Project 2015-2016 Monitoring and Treatment Report Prepared by: Tobias Rohmer Olofson Environmental, Inc. & Drew Kerr 1830 Embarcadero Cove, Suite 100 Oakland, California 94606 For the State Coastal Conservancy San Francisco Estuary Invasive Spartina Project 1330 Broadway, 13th Floor Oakland, CA 94612 April 2018 Cover Photos (clockwise from top left) Biologists frequently use airboats to gain access to marsh interiors, as seen here within Bair Island Ecological Reserve in Redwood City, The range of hybrid Spartina alterniflora expanded as this clone was discovered on the shore of Point Buckler Island in Grizzly Bay. Native Spartina foliosa has established and expanded in several restoration sites of the South Bay Salt Pond Restoration Project, as documented here in Pond A21 along Coyote Creek. This report was prepared for the California Coastal Conservancy’s San Francisco Estuary In- vasive Spartina Project with support and fund- ing from the following contributors: California Coastal Conservancy California Wildlife Conservation Board (MOU #99-054-01 and subsequent) Table of Contents 1. Introduction and Summary of Progress Through 2014 ...................................................... 1 2. Treatment and Monitoring Completed 2015-2016 .............................................................. 3 2.2. Bay-wide Inventory .......................................................................................................................... -
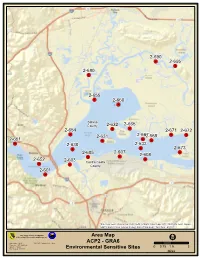
GRA 6 Suisun Bay
2-690 .! 2-695 .! 2-680 .! 2-655 .! 2-660 .! Solano 2-632 2-665 County ! 2-654 .! . 2-671 2-672 .! 2-631 2-6672-668 .! .! 2-651 .! .! .! .! 2-630 2-633 .! .! 2-673 2-607 .! 2-605 2-608 .! .! 2-652 2-603 .! .! Contra Costa .! County 2-601 .! Sources: Esri, DeLorme, NAVTEQ, USGS, Intermap, iPC, NRCAN, Esri Japan, METI, Esri China (Hong Kong), Esri (Thailand), TomTom, 2013 OSPR Calif. Dept. of Fish and Wildlife Office of Spill Prevention and Respon se Area Map Office of Spill Prevention and Response I Data Source: O SPR NAD_1983_C alifornia_Teale_Albers ACP2 - GRA6 Requestor: ACP Coordinator Author: J. Muskat 00.75 1.53 Date Created: 5/12/2014 Environmental Sensitive Sites Miles Section 9846 – GRA 6 Suisun Bay Table of Contents GRA 6 GRA 6 Map ........................................................................................................................... 1 Table of Contents Introduction................................................................................................ 2 Site Index/Response Actions................................................................................................ 3 Summary of Response Resources for GRA 6......................................................................... 4 9846.1 Ecologically Sensitive Sites 2-601 -A Martinez Marsh ............................................................................................ 1 2-603 -A Bulls Head Marsh and Pacheco Creek ..................................................... 5 2-605 -A Hastings Slough, Point Edith and Seal Island.......................................... -

9. Suisun Bay Off Bull's Head Point Near Martinez Samples Are
6. Suisun Bay off Bull’s Head Point near 38-02-40 122-07-00 Martinez Samples are collected near the Southern Pacific Railroad bridge at Benicia. 7. Grizzly Bay at Dolphin near Suisun 38-07-02 122-02-19 Slough Samples are collected from a shallow embayment 1.4 miles east of the mouth of Suisun Slough. 8. Suisun Bay off Middle Point near 38-03-36 121 -59-20 Nichols Samples are collected in Suisun Bay within the west reach of the Middle Ground Channel. 9. Honker Bay near Wheeler point 38-04-26 121-56-1 2 The sampling site is located in a shallow embayment 1.9 miles northeasterly from Point Palo Alto. 10. Sacramento River at Chipps Island 38-02-47 12 1-55-02 Samples are collected west of the confluence of the Sacramento and San Joaquin Rivers between Chipps and Mallard Islands. 11. Sherman Lake near Antioch 38-02-34 12 1-47-34 Samples are collected 2 miles north of Antioch near the center of a submerged tract between the Sacramento and San Joaquin Rivers. 12. San Joaquin River at Antioch. Ship 38-01-1 5 121-48-28 Channel Samples are collected 0.3 miles north of Antioch between the entrance markers of the Antioch Reach Channel in the San Joaquin River. 13. Big Break near Oakley 38-01-05 121-42-38 The sampling site is located 1.3 miles north of Oakley in a submerged tract. 14. San Joaquin River at Jersey Point 38-03-09 121 -41 -1 7 This sampling site is located on the San Joaquin River 6.5 miles northeast of Antioch in the shipping channel. -

National List of Beaches
Contents Introduction ...................................................................................................................................... 1 States Alabama ........................................................................................................................................... 3 Alaska .............................................................................................................................................. 5 California .......................................................................................................................................... 6 Connecticut .................................................................................................................................... 16 Delaware ........................................................................................................................................ 18 Florida ............................................................................................................................................ 19 Georgia .......................................................................................................................................... 31 Hawaii ............................................................................................................................................ 33 Illinois ............................................................................................................................................. 41 Indiana .......................................................................................................................................... -

4.7 LAND USE and RECREATION 1 This Section Describes the Existing Land and Recreational Uses in the Vicinity of the 2 Proposed S
4.7 Land Use and Recreation 1 4.7 LAND USE AND RECREATION 2 This Section describes the existing land and recreational uses in the vicinity of the 3 proposed San Francisco Bay and Delta Sand Mining Project (Project), summarizes 4 applicable land use plans and policies, evaluates Project consistency with these plans 5 and policies, and evaluates the compatibility of the proposed Project with adjacent and 6 surrounding land and recreational uses. 7 4.7.1 Environmental Setting 8 Lease Areas 9 Hanson Marine Operations (Hanson) and Jerico Products/Morris Tug and Barge (Jerico) 10 (the applicants) have applied to the California State Lands Commission (CSLC) for new 11 10-year mineral extraction leases to continue mining sand within certain delineated lease 12 parcels of Central San Francisco Bay (Central Bay), Suisun Bay, and the western 13 Sacramento-San Joaquin River Delta area (Delta).1 The Applicants also propose to 14 continue sand mining of privately owned submerged lands within Suisun Bay (Middle 15 Ground Shoal). 16 Uses of these waters and submerged lands include shipping, commercial and 17 recreational fishing, recreational boating, other water-oriented recreation, and estuarine 18 and wildlife habitat. Dredging occurs regularly within the Bay and Delta navigation 19 channels, and parts of the Bay are used for the disposal of dredging spoils. Central Bay 20 shipping channels overlap several of the mining parcels, and designated underwater 21 cable areas are located adjacent to several of the Central Bay parcels. A shipping 22 channel parallels the Contra Costa County shoreline south of the Middle Ground parcel 23 and the southern end of the Sacramento Deep Water Ship Channel roughly coincides 24 with the eastern boundary of CSLC lease parcel PRC 7781 south of Collinsville. -

20160127 Nishi Development LEED ND V4
Memorandum TO Tim Ruff FROM Abena Darden COMPANY Norcal Land DATE January 27, 2016 LEED for Neighborhood Development RE v4 Prerequisite Assessment – DRAFT PROJECT NO U16019.00 v1.1 PROJECT CC Lynn N. Simon, TT; File Nishi Development NAME Executive Summary Thornton Tomasetti (TT) has been tasked to review the Nishi Development and assess whether it can achieve a LEED for Neighborhood Development (ND) certification. TT’s first step included assessing the LEED for Neighborhood Development v4 prerequisites in the Smart Location & Linkages (SLL) and Neighborhood Pattern and Design (NPD) categories. Thornton Tomasetti did not review the Green Building & Infrastructure prerequisites as the state green building code, CALGreen, and Title 24-2013 energy requirements lend themselves well to complying with the four prerequisites under that category. The report is divided into the two credit categories, SLL and NPD, and includes a breakdown of the prerequisites requirements, analysis of the requirements, and summary of compliance. We have included only the requirements we felt were relevant to the project. For a full list of the requirements, please visit: http://www.usgbc.org/credits/neighborhood-development-plan/v4. Based upon the documents we have been provided (i.e. Sustainability Implementation Plan, and documents found online such as the Environmental impact Report), and discussion with Adam Maynard, a US Green Building Council (USGBC) staff person with the LEED ND team, we have determined that pursuing a LEED for Neighborhood Development certification for the project is not feasible at this time. Presently, the project is not meeting four of the eight evaluated prerequisites. While three of these could be met with design changes and additional effort on behalf of the team and developer, one prerequisite is outside of the control of the project team: SLLp Smart Location.