Identification of a Novel Deletion Mutation in DPY19L2 from An
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in A
Program Nr: 1 from the 2004 ASHG Annual Meeting Mutations in a novel member of the chromodomain gene family cause CHARGE syndrome. L.E.L.M. Vissers1, C.M.A. van Ravenswaaij1, R. Admiraal2, J.A. Hurst3, B.B.A. de Vries1, I.M. Janssen1, W.A. van der Vliet1, E.H.L.P.G. Huys1, P.J. de Jong4, B.C.J. Hamel1, E.F.P.M. Schoenmakers1, H.G. Brunner1, A. Geurts van Kessel1, J.A. Veltman1. 1) Dept Human Genetics, UMC Nijmegen, Nijmegen, Netherlands; 2) Dept Otorhinolaryngology, UMC Nijmegen, Nijmegen, Netherlands; 3) Dept Clinical Genetics, The Churchill Hospital, Oxford, United Kingdom; 4) Children's Hospital Oakland Research Institute, BACPAC Resources, Oakland, CA. CHARGE association denotes the non-random occurrence of ocular coloboma, heart defects, choanal atresia, retarded growth and development, genital hypoplasia, ear anomalies and deafness (OMIM #214800). Almost all patients with CHARGE association are sporadic and its cause was unknown. We and others hypothesized that CHARGE association is due to a genomic microdeletion or to a mutation in a gene affecting early embryonic development. In this study array- based comparative genomic hybridization (array CGH) was used to screen patients with CHARGE association for submicroscopic DNA copy number alterations. De novo overlapping microdeletions in 8q12 were identified in two patients on a genome-wide 1 Mb resolution BAC array. A 2.3 Mb region of deletion overlap was defined using a tiling resolution chromosome 8 microarray. Sequence analysis of genes residing within this critical region revealed mutations in the CHD7 gene in 10 of the 17 CHARGE patients without microdeletions, including 7 heterozygous stop-codon mutations. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

A New AURKC Mutation Causing Macrozoospermia
A new AURKC mutation causing macrozoospermia: implications for human spermatogenesis and clinical diagnosis Mariem Ben Khelifa 1 2 3 , Raoudha Zouari 4 , Radu Harbuz 2 1 , Lazhar Halouani 4 , Christophe Arnoult 1 , Joël Lunardi 2 5 , Pierre F. Ray 1 2 * 1 AGIM, AGeing and IMagery, CNRS FRE3405 Université Joseph Fourier - Grenoble I , Ecole Pratique des Hautes Etudes , CNRS : UMR5525 , Faculté de médecine de Grenoble, 38700 La Tronche,FR 2 Laboratoire de biochimie et génétique moléculaire CHU Grenoble , 38043 Grenoble,FR 3 Molecular Investigation of Genetic Orphan Diseases Research Unit Institut Pasteur de Tunis , Research Unit UR04/SP03,TN 4 Clinique de la reproduction les Jasmins Clinique de la reproduction les Jasmins , 23, Av. Louis BRAILLE, 1002 Tunis,TN 5 GIN, Grenoble Institut des Neurosciences INSERM : U836 , CEA , Université Joseph Fourier - Grenoble I , CHU Grenoble , UJF - Site Santé La Tronche BP 170 38042 Grenoble Cedex 9,FR * Correspondence should be addressed to: Pierre Ray <[email protected] > Abstract The presence of close to 100% large-headed multi-tailed spermatozoa in the ejaculate has been described as a rare phenotype of male infertility with a very poor prognosis. We demonstrated previously that most cases were caused by a homozygous mutation (c.144delC) in the Aurora Kinase C gene (AURKC) leading to the absence or the production of a nonfunctional protein. AURKC deficiency in these patients blocked meiosis and resulted in the production of tetraploid spermatozoa unsuitable for fertilization. We describe here the study of two brothers presenting with large-headed spermatozoa. Molecular analysis of the AURKC gene was carried out in two brothers presenting with a typical large headed spermatozoa phenotype. -

Globozoospermia Syndrome: an Update
Received: 16 July 2019 | Revised: 17 September 2019 | Accepted: 21 September 2019 DOI: 10.1111/and.13459 INVITED REVIEW Globozoospermia syndrome: An update Farzaneh Fesahat1 | Ralf Henkel2,3 | Ashok Agarwal3 1Reproductive Immunology Research Center, Shahid Sadoughi University of Abstract Medical Sciences, Yazd, Iran Among the factors involved in male infertility, there is a rare morphology disorder 2 Department of Medical called "globozoospermia" that is classified into total globozoospermia and partial Bioscience, University of the Western Cape, Bellville, South Africa globozoospermia (type I and type II, respectively). This syndrome is primarily char‐ 3American Center for Reproductive acterised by the presence of round‐headed spermatozoa with cytoskeleton defects Medicine, Cleveland Clinic, Cleveland, OH, USA around the nucleus and no acrosome. Current data support the negative correlation between globozoospermia and conventional intracytoplasmic sperm injection (ICSI) Correspondence Ashok Agarwal, American Center for outcomes, revealing the need for the management of patients undergoing assisted Reproductive Medicine, Cleveland Clinic, reproduction technology (ART) through more effective treatment techniques. This Cleveland, OH, USA. Email: [email protected] review highlights the most important characteristics of globozoospermia such as sperm parameters, DNA/chromatin integrity and sperm DNA fragmentation (SDF), as well as genetic features based on the latest knowledge. Additionally, we looked into current progress on fertilisation potential and possible treatment strategies for patients presenting with globozoospermia. KEYWORDS DNA fragmentation, globozoospermia, human, intracytoplasmic sperm injection, morphology, spermatozoa 1 | INTRODUCTION causes primary male infertility (Singh, 1992). Contrary, men with type II globozoospermia have both normal and round‐headed sperm Among the factors involved in male infertility, there is a rare mor‐ cells with large CDs, which impair motility. -

Supplemental Table 3 Two-Class Paired Significance Analysis of Microarrays Comparing Gene Expression Between Paired
Supplemental Table 3 Two‐class paired Significance Analysis of Microarrays comparing gene expression between paired pre‐ and post‐transplant kidneys biopsies (N=8). Entrez Fold q‐value Probe Set ID Gene Symbol Unigene Name Score Gene ID Difference (%) Probe sets higher expressed in post‐transplant biopsies in paired analysis (N=1871) 218870_at 55843 ARHGAP15 Rho GTPase activating protein 15 7,01 3,99 0,00 205304_s_at 3764 KCNJ8 potassium inwardly‐rectifying channel, subfamily J, member 8 6,30 4,50 0,00 1563649_at ‐‐ ‐‐ ‐‐ 6,24 3,51 0,00 1567913_at 541466 CT45‐1 cancer/testis antigen CT45‐1 5,90 4,21 0,00 203932_at 3109 HLA‐DMB major histocompatibility complex, class II, DM beta 5,83 3,20 0,00 204606_at 6366 CCL21 chemokine (C‐C motif) ligand 21 5,82 10,42 0,00 205898_at 1524 CX3CR1 chemokine (C‐X3‐C motif) receptor 1 5,74 8,50 0,00 205303_at 3764 KCNJ8 potassium inwardly‐rectifying channel, subfamily J, member 8 5,68 6,87 0,00 226841_at 219972 MPEG1 macrophage expressed gene 1 5,59 3,76 0,00 203923_s_at 1536 CYBB cytochrome b‐245, beta polypeptide (chronic granulomatous disease) 5,58 4,70 0,00 210135_s_at 6474 SHOX2 short stature homeobox 2 5,53 5,58 0,00 1562642_at ‐‐ ‐‐ ‐‐ 5,42 5,03 0,00 242605_at 1634 DCN decorin 5,23 3,92 0,00 228750_at ‐‐ ‐‐ ‐‐ 5,21 7,22 0,00 collagen, type III, alpha 1 (Ehlers‐Danlos syndrome type IV, autosomal 201852_x_at 1281 COL3A1 dominant) 5,10 8,46 0,00 3493///3 IGHA1///IGHA immunoglobulin heavy constant alpha 1///immunoglobulin heavy 217022_s_at 494 2 constant alpha 2 (A2m marker) 5,07 9,53 0,00 1 202311_s_at -

Human Genetics of Male Infertility Elias Elinati
Human genetics of male infertility Elias Elinati To cite this version: Elias Elinati. Human genetics of male infertility. Genomics [q-bio.GN]. Université de Strasbourg, 2012. English. NNT : 2012STRAJ120. tel-00872193 HAL Id: tel-00872193 https://tel.archives-ouvertes.fr/tel-00872193 Submitted on 11 Oct 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITÉ DE STRASBOURG ÉCOLE DOCTORALE des Sciences de la Vie et de la Santé IGBMC - CNRS UMR 7104 - Inserm U 964 THÈSE présentée par : Elias ELINATI soutenue le : 10 Septembre 2012 pour obtenir le grade de : Docteur de l’Université de Strasbourg Discipline/ Spécialité : Aspects moléculaires et cellulaires de la biologie TITRE de la thèse Génétique de l’infertilité masculine THÈSE dirigée par : M. VIVILLE Stéphane Professeur, Université de Strasbourg RAPPORTEURS : Mme CHABOISSIER Marie-Christine Docteur, Institut de Biologoie Valrose M. TURNER James Docteur, National institute of medical research AUTRES MEMBRES DU JURY : M KOENIG Michel Professeur, Université de Strasbourg The learning and knowledge that we have, is, at the most, but little compared with that of which we are ignorant. Plato Acknowledgments Finally, it’s time for me to graduate … Yeah, I did it! While I am enthousiastic to finally bring a chapter of my life to a close, I have to say it has changed me on all levels and allowed me to meet many outstanding persons. -

Investigations Génétiques D'une Large Cohorte De Patients Infertiles Avec
Investigations génétiques d’une large cohorte de patients infertiles avec globozoospermie, implication du gène DPYL19L2 et proposition d’une nouvelle stratégie de diagnostic génétique Tristan Celse To cite this version: Tristan Celse. Investigations génétiques d’une large cohorte de patients infertiles avec globozoosper- mie, implication du gène DPYL19L2 et proposition d’une nouvelle stratégie de diagnostic génétique. Médecine humaine et pathologie. 2020. dumas-03027534 HAL Id: dumas-03027534 https://dumas.ccsd.cnrs.fr/dumas-03027534 Submitted on 27 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance. La propriété intellectuelle du document reste entièrement celle du ou des auteurs. Les utilisateurs doivent respecter le droit d’auteur selon la législation en vigueur, et sont soumis aux règles habituelles du bon usage, comme pour les publications sur papier : respect des travaux originaux, citation, interdiction du pillage intellectuel, etc. Il est mis à disposition de toute personne intéressée par l’intermédiaire de l’archive ouverte DUMAS (Dépôt Universitaire de Mémoires Après Soutenance). Si vous désirez contacter son ou ses auteurs, nous vous invitons à consulter la page de DUMAS présentant le document. -
Exome Sequencing Reveals Novel Causes As Well As New Candidate Genes for Human Globozoospermia
Human Reproduction, pp. 1–13, 2020 doi:10.1093/humrep/dez246 ORIGINAL ARTICLE Reproductive genetics Downloaded from https://academic.oup.com/humrep/advance-article-abstract/doi/10.1093/humrep/dez246/5715929 by University of Newcastle user on 29 January 2020 Exome sequencing reveals novel causes as well as new candidate genes for human globozoospermia M.S. Oud1, Ö. Okutman2,3, L.A.J. Hendricks1,P.F.deVries1, B.J. Houston4, L.E.L.M. Vissers1, M.K. O’Bryan4, L. Ramos5, H.E. Chemes6,S.Viville2,3,†, and J.A. Veltman1,7,*,† 1Department of Human Genetics, Donders Institute for Brain, Cognition and Behavior, Radboudumc, Nijmegen, The Netherlands 2Laboratoire de Diagnostic Génétique, UF3472-génétique de l’infertilité, Hôpitaux Universitaires de Strasbourg, 67000 Strasbourg, France 3Institut de Parasitologie et Pathologie Tropicale, EA 7292, Université de Strasbourg, 3 rue Koeberlé, 67000 Strasbourg, France 4School of Biological Sciences, Monash University, Clayton, Australia 5Department of Gynaecology and Obstetrics, Radboudumc, Nijmegen, The Netherlands 6Center for Research in Endocrinology (CEDIE), National Research Council, Department of Endocrinology, Buenos Aires Children’s Hospital, Argentina 7Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, UK * Correspondence address. E-mail: [email protected] Submitted on July 15, 2019; resubmitted on October 8, 2019; editorial decision on October 18, 2019 STUDY QUESTION: Can exome sequencing identify new genetic causes of globozoospermia? SUMMARY ANSWER: Exome sequencing in 15 cases of unexplained globozoospermia revealed deleterious mutations in seven new genes, of which two have been validated as causing globozoospermia when knocked out in mouse models. WHAT IS KNOWN ALREADY: Globozoospermia is a rare form of male infertility characterised by round-headed sperm and malformation of the acrosome. -
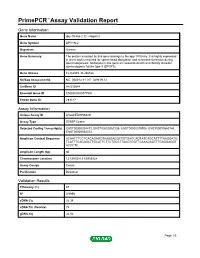
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name dpy-19-like 2 (C. elegans) Gene Symbol DPY19L2 Organism Human Gene Summary The protein encoded by this gene belongs to the dpy-19 family. It is highly expressed in testis and is required for sperm head elongation and acrosome formation during spermatogenesis. Mutations in this gene are associated with an infertility disorder spermatogenic failure type 9 (SPGF9). Gene Aliases FLJ32949, FLJ36166 RefSeq Accession No. NC_000012.11, NT_029419.12 UniGene ID Hs.533644 Ensembl Gene ID ENSG00000177990 Entrez Gene ID 283417 Assay Information Unique Assay ID qHsaCED0056829 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000324472, ENST00000262135, ENST00000379925, ENST00000563746, ENST00000564374 Amplicon Context Sequence ACAATTTCCTCACAGAGGTAAAGAGGCTGTGACCAGTATCAGCATTTTAAGGATG TCATTTCAGAGCTTGCATTCTTCTGCCTTAAGTGGTTCAAAGAGTTTGAGGAGGT ACCCTC Amplicon Length (bp) 86 Chromosome Location 12:63953813-63953928 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 97 R2 0.9996 cDNA Cq 26.34 cDNA Tm (Celsius) 79 gDNA Cq 24.52 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report DPY19L2, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 3/5 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. -

DPY19L2 Gene Dpy-19 Like 2
DPY19L2 gene dpy-19 like 2 Normal Function The DPY19L2 gene provides instructions for making a protein that is found in developing sperm cells. The DPY19L2 protein plays a role in the development of the acrosome, a cap-like structure in the head of sperm cells. The acrosome contains enzymes that break down the outer membrane of egg cells, allowing the sperm to fertilize an egg. The developing acrosome is attached to the nucleus of the cell. The DPY19L2 protein, which is found within the membrane of the nucleus, helps attach the forming acrosome to the nuclear membrane. As the acrosome develops and the sperm cell matures, the acrosome moves to the tip of the head of the sperm, which helps the head elongate into an oval shape. Health Conditions Related to Genetic Changes Globozoospermia At least 17 DPY19L2 gene mutations have been found in men with globozoospermia, a condition characterized by abnormal sperm cells that have a round head and no acrosome. Approximately 70 percent of men with this condition have mutations in DPY19L2. Most of these mutations delete large regions of the gene or the whole gene. Others change single protein building blocks (amino acids) in the DPY19L2 protein. These mutations lead to a loss of functional DPY19L2 protein. Without this protein, the forming acrosome is not attached to the nucleus and is removed from the cell. As a result, sperm cells have no acrosome and the head of the sperm does not elongate. The abnormal sperm are unable to get through the outer membrane of an egg cell to fertilize it, leading to an inability to father biological children (infertility) in affected men. -

Genetic Investigation in Non-Obstructive Azoospermia: from the X Chromosome to The
DOTTORATO DI RICERCA IN SCIENZE BIOMEDICHE CICLO XXIX COORDINATORE Prof. Persio dello Sbarba Genetic investigation in non-obstructive azoospermia: from the X chromosome to the whole exome Settore Scientifico Disciplinare MED/13 Dottorando Tutore Dott. Antoni Riera-Escamilla Prof. Csilla Gabriella Krausz _______________________________ _____________________________ (firma) (firma) Coordinatore Prof. Carlo Maria Rotella _______________________________ (firma) Anni 2014/2016 A la meva família Agraïments El resultat d’aquesta tesis és fruit d’un esforç i treball continu però no hauria estat el mateix sense la col·laboració i l’ajuda de molta gent. Segurament em deixaré molta gent a qui donar les gràcies però aquest són els que em venen a la ment ara mateix. En primer lloc voldria agrair a la Dra. Csilla Krausz por haberme acogido con los brazos abiertos desde el primer día que llegué a Florencia, por enseñarme, por su incansable ayuda y por contagiarme de esta pasión por la investigación que nunca se le apaga. Voldria agrair també al Dr. Rafael Oliva per haver fet possible el REPROTRAIN, per haver-me ensenyat moltíssim durant l’època del màster i per acceptar revisar aquesta tesis. Alhora voldria agrair a la Dra. Willy Baarends, thank you for your priceless help and for reviewing this thesis. També voldria donar les gràcies a la Dra. Elisabet Ars per acollir-me al seu laboratori a Barcelona, per ajudar-me sempre que en tot el què li he demanat i fer-me tocar de peus a terra. Ringrazio a tutto il gruppo di Firenze, che dal primo giorno mi sono trovato come a casa. -
Transcriptome Profiling of Developing Testes and Spermatogenesis in The
Li et al. BMC Genetics (2020) 21:46 https://doi.org/10.1186/s12863-020-00843-5 RESEARCH ARTICLE Open Access Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse Bei Li1,2,3, Xiaolong He4, Yiping Zhao1,2,3, Dongyi Bai1,2,3, Ming Du1,2,3, Lianjie Song1,2,3, Zhuang Liu1,2,3, Zhenchen Yin1,2,3 and Dugarjaviin Manglai1,2,3* Abstract Background: Horse testis development and spermatogenesis are complex physiological processes. Methods: To study these processes, three immature and three mature testes were collected from the Mongolian horse, and six libraries were established using high-throughput RNA sequencing technology (RNA-Seq) to screen for genes related to testis development and spermatogenesis. Results: A total of 16,237 upregulated genes and 8,641 downregulated genes were detected in the testis of the Mongolian horse. These genes play important roles in different developmental stages of spermatogenesis and testicular development. Five genes with alternative splicing events that may influence spermatogenesis and development of the testis were detected. GO (Gene ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses were performed for functional annotation of the differentially expressed genes. Pathways related to “spermatogenesis,” male gamete generation,”“spermatid development” and “oocyte meiosis” were significantly involved in different stages of testis development and spermatogenesis. Conclusion: Genes, pathways and alternative splicing events were identified with inferred functions in the process of spermatogenesis in the Mongolian horse. The identification of these differentially expressed genetic signatures improves our understanding of horse testis development and spermatogenesis. Keywords: Mongolian horse, Developing testis, Spermatogenesis, RNA-Seq Background meiosis of spermatocytes, and (3) sperm maturation.