Differentiation of Hemorrhage from Iodinated Contrast in Different Intracranial Compartments Using Dual-Energy Head CT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Xx250 Spc 2015 Uk
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT XENETIX 250 (250 mgI/ml) Solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION per ml 50 ml 100 ml 200 ml 500 ml Iobitridol (INN) 548.4 mg 27.42 g 54.84 g 109.68 g 274.2 g Iodine corresponding to 250 mg 12.5 g 25 g 50 g 125 g Excipient with known effect : Sodium (up to 3.5 mg per 100 mL). For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection. Clear, colourless to pale yellow solution 4. CLINICAL PARTICULARS 4.1. Therapeutic indications For adults and children undergoing: . whole-body CT . venography . intra-arterial digital subtraction angiography . ERP/ERCP This medicinal product is for diagnostic use only. 4.2. Posology and method of administration The dosage may vary depending on the type of examination, the age, weight, cardiac output and general condition of the patient and the technique used. Usually the same iodine concentration and volume are used as with other iodinated X-ray contrast in current use. As with all contrast media, the lowest dose necessary to obtain adequate visualisation should be used. Adequate hydration should be assured before and after administration as for other contrast media. As a guideline, the recommended dosages are as follows: Indications Recommended dosage Whole-body CT The doses of contrast medium and the rates of administration depend on the organs under investigation, the diagnostic problem and, in particular, the different scan and image-reconstruction times of the scanners in use. -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

"Fullness" of the Pancreas on CT. Usually Benign, but EUS Plus/Minus FNA Is Warranted to Identify Malignancy
JOP. J Pancreas (Online) 2009 Jan 8; 10(1):37-42. ORIGINAL ARTICLE Focal or Diffuse "Fullness" of the Pancreas on CT. Usually Benign, but EUS Plus/Minus FNA Is Warranted to Identify Malignancy John David Horwhat1, Henning Gerke2, Ruben Daniel Acosta1, Darren A Pavey3, Paul S Jowell3 1Gastroenterology Service, Department of Medicine, Walter Reed Army Medical Center. Washington, DC, USA. 2Division of Gastroenterology, Department of Medicine, University of Iowa Hospitals and Clinics. Iowa City, IA, USA. 3Division of Gastroenterology, Department of Medicine, Duke University Health System. Durham, NC, USA ABSTRACT Context The role of EUS to evaluate subtle radiographic abnormalities of the pancreas is not well defined. Objective To assess the yield of EUS±FNA for focal or diffuse pancreatic enlargement/fullness seen on abdominal CT scan in the absence of discrete mass lesions. Design Retrospective database review. Setting Tertiary referral center. Patients and interventions Six hundred and 91 pancreatic EUS exams were reviewed. Sixty-nine met inclusion criteria of having been performed for focal enlargement or fullness of the pancreas. Known chronic pancreatitis, pancreatic calcifications, acute pancreatitis, discrete mass on imaging, pancreatic duct dilation (greater than 4 mm) and obstructive jaundice were excluded. Main outcome measurement Rate of malignancy found by EUS±FNA. Results FNA was performed in 19/69 (27.5%) with 4 new diagnoses of pancreatic adenocarcinoma, one metastatic renal cell carcinoma, one metastatic colon cancer, one chronic pancreatitis and 12 benign results. Eight patients had discrete mass lesions on EUS; two were cystic. All malignant diagnoses had a discrete solid mass on EUS. Conclusions Pancreatic enlargement/fullness is often a benign finding related to anatomic variation, but was related to malignancy in 8.7% of our patients (6/69). -

Review Article Contrast Media Viscosity Versus Osmolality in Kidney Injury: Lessons from Animal Studies
Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 358136, 15 pages http://dx.doi.org/10.1155/2014/358136 Review Article Contrast Media Viscosity versus Osmolality in Kidney Injury: Lessons from Animal Studies Erdmann Seeliger, Diana C. Lenhard, and Pontus B. Persson Institute of Physiology and Center for Cardiovascular Research, Charite-University´ Medicine Berlin, Campus Mitte, Hessische Straße 3-4, 10115 Berlin, Germany Correspondence should be addressed to Erdmann Seeliger; [email protected] Received 18 October 2013; Accepted 29 December 2013; Published 23 February 2014 Academic Editor: Richard Solomon Copyright © 2014 Erdmann Seeliger et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Iodinated contrast media (CM) can induce acute kidney injury (AKI). CM share common iodine-related cytotoxic features but differ considerably with regard to osmolality and viscosity. Meta-analyses of clinical trials generally failed to reveal renal safety differences of modern CM with regard to these physicochemical properties. While most trials’ reliance on serum creatinine as outcome measure contributes to this lack of clinical evidence, it largely relies on the nature of prospective clinical trials: effective prophylaxis by ample hydration must be employed. In everyday life, patients are often not well hydrated; here we lack clinical data. However, preclinical studies that directly measured glomerular filtration rate, intrarenal perfusion and oxygenation, and various markers of AKI have shown that the viscosity of CM is of vast importance. In the renal tubules, CM become enriched, as water is reabsorbed, but CM are not. -

When Is the Use of Contrast Media in Chest CT Indicated? Bruno Hochhegger1,2,3, Robson Rottenfusser4,5, Edson Marchiori6
J Bras Pneumol. 2017;43(5):400-400 http://dx.doi.org/10.1590/S1806-37562017000000179 LETTER TO THE EDITOR When is the use of contrast media in chest CT indicated? Bruno Hochhegger1,2,3, Robson Rottenfusser4,5, Edson Marchiori6 TO THE EDITOR: nodule, small or large airway disease, and lung cancer screening, do not require the use of any contrast media. It is undeniable that CT plays an important role in the diagnosis and treatment of various clinical conditions However, for the assessment of vascular disease, CT involving the chest wall, mediastinum, pleura, pulmonary requires the use of an i.v. contrast agent to delineate the arteries, and lung parenchyma. The need for enhancement vessel lumen (e.g., aneurysm, dissection, and vascular using i.v. contrast media depends on the specific clinical tumor invasion). Pulmonary embolic disease is the third indication.(1-3) The most common contrast agents used most common cause of acute cardiovascular disease.(3,5) with CT imaging are iodinated. Intravenous iodinated Chest CT angiography is the most common way to assess contrast agents are used for opacification of vascular for pulmonary embolic disease because, in addition to structures. The major families of contrast agents are being accurate, fast, and widely available, it can assess ionic and nonionic. Contrast agents can be further alternate pathologies in cases of undifferentiated chest classified as high- or low-osmolality agents on the basis pain. Typically, the use of CT in order to investigate of the iodine concentration. Most centers use nonionic vascular disease or pleural pathology may include contrast agents (generally low-osmolality agents) for non-contrast-enhanced images to identify hemorrhage, i.v. -

Coronary Artery Calcium Quantification in Contrast-Enhanced Computed Tomography Angiography
Georgia State University ScholarWorks @ Georgia State University Computer Science Dissertations Department of Computer Science 12-18-2013 Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography Abinashi Dhungel Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/cs_diss Recommended Citation Dhungel, Abinashi, "Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography." Dissertation, Georgia State University, 2013. https://scholarworks.gsu.edu/cs_diss/80 This Dissertation is brought to you for free and open access by the Department of Computer Science at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Computer Science Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. CORONARY ARTERY CALCIUM QUANTIFICATION IN CONTRAST-ENHANCED COMPUTED TOMOGRAPHY ANGIOGRAPHY by ABINASHI DHUNGEL Under the Direction of Dr. Michael Weeks ABSTRACT Coronary arteries are the blood vessels supplying oxygen-rich blood to the heart muscles. Coronary artery calcium (CAC), which is the total amount of calcium deposited in these arteries, indicates the presence or the future risk of coronary artery diseases. Quantification of CAC is done by using computed tomography (CT) scan which uses attenuation of x- ray by different tissues in the body to generate three-dimensional images. Calcium can be easily spotted in the CT images because of its higher opacity to x-ray compared to that of the surrounding tissue. However, the arteries cannot be identified easily in the CT images. Therefore, a second scan is done after injecting a patient with an x-ray opaque dye known as contrast material which makes different chambers of the heart and the coronary arteries visible in the CT scan. -

Iodinated Contrast Media and Their Adverse Reactions*
Iodinated Contrast Media and Their Adverse Reactions* Jagdish Singh and Aditya Daftary Teleradiology Solutions, Bangalore, India acteristics, including their chemical structure, osmolality, Cross-use of technology between nuclear medicine and radiol- iodine content, and ionization in solution (2,3). ogy technologists is expanding. The growth of PET/CT and the The parent molecule from which the contrast agents are increasing use of intravenous contrast agents during these pro- derived is benzene. This is a toxic water-insoluble liquid. cedures bring the nuclear medicine technologist into direct con- The carbon atoms on a benzene ring are numbered clock- tact with these agents and their associated complications. A basic understanding of the occurrence, risk factors, clinical fea- wise from 1 to 6. Benzoic acid is produced by introducing tures, and management of these procedures is of increasing an acid group at position 1 on the benzene ring. This acid importance to the nuclear medicine technologist. After reading group permits the formation of salts or amides, which this article, the technologist will be able to list the factors that influence water solubility. 2,4,6-triiodobenzoic acid is ob- increase the risk of contrast reactions; understand ways to min- tained by introducing iodine atoms at positions 2, 4, and 6 imize the occurrence of contrast reactions; and develop a plan to on the ring. Iodine is the element used in contrast media identify, treat, and manage the reactions effectively. as it possesses 3 important properties essential for the Key Words: iodinated contrast media; contrast reactions production of contrast media: high-contrast density, firm J Nucl Med Technol 2008; 36:69–74 binding to the benzene molecule, and low toxicity. -

Iodinated Contrast Media Guideline
IODINATED CONTRAST MEDIA GUIDELINE FACULTY OF CLINICAL RADIOLOGY THE ROYAL AUSTRALIAN AND NEW ZEALAND COLLEGE OF RADIOLOGISTS® Name of document and version: Iodinated Contrast Media Guideline, V2.3 Approved by: Faculty of Clinical Radiology Date of approval: March 2018 Suggested Citation: The Royal Australian and New Zealand College of Radiologists. Iodinated Contrast Media Guideline. Sydney: RANZCR; 2018. ABN 37 000 029 863 Copyright for this publication rests with The Royal Australian and New Zealand College of Radiologists ® The Royal Australian and New Zealand College of Radiologists Level 9, 51 Druitt Street Sydney NSW 2000 Australia Email: [email protected] Website: www.ranzcr.edu.au Telephone: +61 2 9268 9777 Disclaimer: The information provided in this document is of a general nature only and is not intended as a substitute for medical or legal advice. It is designed to support, not replace, the relationship that exists between a patient and his/her doctor. Document name Iodinated Contrast Media Guideline Description The Iodinated Contrast Media Guideline is intended to assist The Royal Australian and New Zealand College of Radiologists®, its staff, Fellows, members and other individuals involved in the administration of iodinated contrast media to patients undergoing medical imaging procedures. Created By Standards of Practice and Accreditation Committee Date Created 2011 Maintained By Faculty of Clinical Radiology, Policy and Advocacy Unit Version Number Modifications Made Date Modified 2011 Document published 2.0 Document updated and published April 2016 2.1 Grammatical error corrected in R31 February 2017 2.2 Nitroglycerin dosages corrected in R39 June 2017 2.3 Adrenaline dosage corrected in R56 March 2018 Iodinated Contrast Media Guideline, V2.3 © The Royal Australian and New Zealand College of Radiologists® March 2018 Page 2 of 39 TABLE OF CONTENTS 1. -

(320 Mg I/Ml), Solution for Injection Meglumine Ioxaglate and Sodium Ioxaglate
HEXABRIX 320 (320 mg I/mL), solution for injection Meglumine ioxaglate and sodium ioxaglate Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor or pharmacist. This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. In this leaflet: 1. What HEXABRIX 320, solution for injection is and what it is used for 2. What you need to know before you use HEXABRIX 320, solution for injection 3. How to use HEXABRIX 320, solution for injection 4. Possible side effects 5. How to store HEXABRIX 320, solution for injection 6. Contents of the pack and other information 1. WHAT HEXABRIX 320 IS AND WHAT IT IS USED FOR HEXABRIX 320 is a contrast agent. It belongs to the group of contrast agents used for radiological examinations. HEXABRIX 320 is used to enhance the contrast of the images obtained during these examinations, in order to improve the visualisation and delineation of certain parts of the body, such as: Certain hollow organs (opacification of body cavities), The uterus and fallopian tubes (ducts connecting the uterus to the ovaries) in female patients (hysterosalpingography), Blood vessels (angiography), Joints (arthrography), Urinary system (urography). This medicine is for diagnostic use only. 2. -
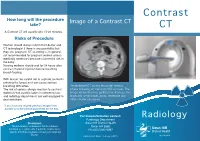
Contrast CT Take? CT a Contrast CT Will Usually Take 15-20 Minutes
Contrast How long will the procedure Image of a Contrast CT take? CT A Contrast CT will usually take 15-20 minutes. Risks of Procedure Women should always inform their doctor and CT technologist if there is any possibility that they are pregnant. CT scanning is, in general, not recommended for pregnant women unless medically necessary because of potential risk to the baby. Nursing mothers should wait for 24 hours after contrast material injection before resuming breast-feeding. With Ioscan, be careful not to aspirate (contents entering the lungs) as it can cause serious breathing difficulties. An abdomen CT during the portal-venous The risk of serious allergic reaction to contrast phase following an injection of IV contrast. The materials that contain iodine is extremely low, image shows the liver, gallbladder, kidneys, the and radiology departments are well-equipped to large and small bowel, aorta, vertebrae and deal with them. other smaller structures. If you have any related previous images from another provider please bring them on the day. For more information contact: Radiology Radiology Department Disclaimer: Swan Hill District Health The information contained in this brochure is Swan Hill 3585 intended as a guide only. If patients require more Ph: (03) 5033 9287 specific information please contact your referring Doctor. Publication Date: February 2013 What is a Contrast CT? Preparation CT scans of internal organs, bones, soft tissue Women must inform the radiographer if there is If you are over 55 years of age, your doctor will and blood vessels reveal more details than any chance that they are pregnant or if they are give you a pathology slip to have a ‘U & E’ blood regular x-rays. -

Delayed Cutaneous Reactions to Iodinated Contrast
CASE REPORT Delayed Cutaneous Reactions to Iodinated Contrast Daniel R. Mazori, MD; Arielle R. Nagler, MD; Miriam Keltz Pomeranz, MD agents were used: intravenous iohexol and oral diatri- PRACTICE POINTS zoate meglumine–diatrizoate sodium. The eruption was • Delayed cutaneous reactions to iodinated contrast not preceded by fever, malaise, sore throat, rhinorrhea, (IC) are common, but patients frequently are mis- cough, arthralgia, headache, diarrhea, or new medication diagnosed and inadvertently readministered the or supplement use. The patient denied any history of drug offending agent. allergy or cutaneous eruptions. Her current medications, • The most common IC-induced delayed reactions are which she had been taking long-term, were aspirin, self-limited exanthematous eruptions that develop lisinopril, diltiazem, levothyroxine, esomeprazole, parox- within 1 week of exposure. etine, gabapentin,copy and diphenhydramine. • Risk factors for delayed reactions to IC include Physical examination was notable for erythema- atopy, contrast exposure during high pollen season, tous, blanchable, nontender macules coalescing into use of the agent iodixanol, a history of other cutane- patches on the trunk and bilateral arms (Figure). ous drug eruptions, elevated serum creatinine levels, There was slight erythema on the nasolabial folds and and treatment with recombinant interleukin 2. ears.not The mucosal surfaces and distal legs were clear. • Dermatologists can help prevent recurrent reactions The patient was afebrile. Her white blood cell count in patients who require repeated exposure to IC by was 12.5×109/L with 32.3% eosinophils (baseline: white recommending gadolinium-based contrast agents blood cell count, 14.8×109/L; 22% eosinophils)(reference and/or premedication. Dorange, 4.8–10.8×109/L; 1%–4% eosinophils). -

OSMOVIST Iotrolan Injection
PRODUCT MONOGRAPH ® OSMOVIST Iotrolan Injection ® OSMOVIST 240 Iotrolan Injection 240 mg I/mL ® OSMOVIST 300 Iotrolan Injection 300 mg I/mL Dimeric Non-ionic Radiographic Contrast Medium For Myelography Bayer Inc. Date of Preparation: 77 Belfield Road April 11, 2007 Toronto, Ontario M9W 1G6 Canada www.bayer.ca Submission Control No.: 113334 © 2007, Bayer Inc. PRODUCT MONOGRAPH OSMOVIST® Iotrolan injection ® OSMOVIST 240 Iotrolan injection 240 mg I/mL ® OSMOVIST 300 Iotrolan injection 300 mg I/mL THERAPEUTIC CLASSIFICATION A Dimeric Non-ionic Radiographic Contrast Medium For Myelography ACTION and CLINICAL PHARMACOLOGY OSMOVIST (iotrolan) is a dimeric, non-ionic, hexaiodinated radiocontrast medium. The contrast-giving substance, iotrolan, is a dimer of triiodinated isophthalic acid derivatives, in which the firmly bound iodine absorbs the X-rays. Solutions of OSMOVIST 240 mg I/mL and 300 mg I/mL are isotonic to plasma and cerebrospinal fluid at radiologically useful iodine levels. After intrathecal administration, iotrolan is absorbed from cerebrospinal fluid (CSF) into the blood stream. The mean half-life of the transfer of iotrolan from the spinal fluid to the blood plasma was found to be 5.7 ± 6.0 hours in adults. The highest concentration of iodine in plasma occurred 3.5 ± 3.1 hours after intrathecal administration. Plasma protein binding at a concentration of 1.2 mg I/mL is 2.4%. The mean elimination half- Page 2 life of iotrolan in the plasma is 13.6 ± 13.9 hours (median of 9 hours). Approximately 50- 80% of the administered dose is excreted unmetabolized by the kidneys within 24 hours after administration, and approximately 90% within 72 hours.