Computed Tomography for Diagnosing Pulmonary Embolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oxygen and Oxygen Equipment Local Coverage Determination (LCD)
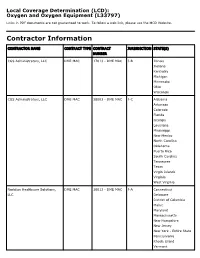
Local Coverage Determination (LCD): Oxygen and Oxygen Equipment (L33797) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER CGS Administrators, LLC DME MAC 17013 - DME MAC J-B Illinois Indiana Kentucky Michigan Minnesota Ohio Wisconsin CGS Administrators, LLC DME MAC 18003 - DME MAC J-C Alabama Arkansas Colorado Florida Georgia Louisiana Mississippi New Mexico North Carolina Oklahoma Puerto Rico South Carolina Tennessee Texas Virgin Islands Virginia West Virginia Noridian Healthcare Solutions, DME MAC 16013 - DME MAC J-A Connecticut LLC Delaware District of Columbia Maine Maryland Massachusetts New Hampshire New Jersey New York - Entire State Pennsylvania Rhode Island Vermont CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, DME MAC 19003 - DME MAC J-D Alaska LLC American Samoa Arizona California - Entire State Guam Hawaii Idaho Iowa Kansas Missouri - Entire State Montana Nebraska Nevada North Dakota Northern Mariana Islands Oregon South Dakota Utah Washington Wyoming LCD Information Document Information LCD ID Original Effective Date L33797 For services performed on or after 10/01/2015 LCD Title Revision Effective Date Oxygen and Oxygen Equipment For services performed on or after 08/02/2020 Proposed LCD in Comment Period Revision Ending Date N/A N/A Source Proposed LCD Retirement Date DL33797 N/A AMA CPT / ADA CDT / AHA NUBC Copyright Notice Period Start Date Statement 06/18/2020 CPT codes, descriptions and other data only are copyright 2019 American Medical Association. All Rights Notice Period End Date Reserved. -

Chronic Cough, Shortness of Breathe, Wheezing? What You Should
CHRONIC COUGH, SHORTNESS OF BREATH, WHEEZING? WHAT YOU SHOULD KNOW ABOUT COPD. A quick guide on Chronic Obstructive Pulmonary Disease COPD.nhlbi.nih.gov COPD, or chronic obstructive Most often, COPD occurs in people pulmonary disease, is a serious lung age 40 and over who… disease that over time makes it hard • Have a history of smoking to breathe. Other names for COPD include • Have had long-term exposure to lung irritants chronic bronchitis or emphysema. such as air pollution, chemical fumes, or dust from the environment or workplace COPD, a leading cause of death, • Have a rare genetic condition called alpha-1 affects millions of Americans and causes antitrypsin (AAT) deficiency long-term disability. • Have a combination of any of the above MAJOR COPD RISK FACTORS history of SMOKING AGE 40+ RARE GENETIC CONDITION alpha-1 antitrypsin (AAT) deficiency LONG-TERM exposure to lung irritants WHAT IS COPD? To understand what COPD is, we first The airways and air sacs are elastic (stretchy). need to understand how respiration When breathing in, each air sac fills up with air like and the lungs work: a small balloon. When breathing out, the air sacs deflate and the air goes out. When air is breathed in, it goes down the windpipe into tubes in the lungs called bronchial In COPD, less air flows in and out of tubes or airways. Within the lungs, bronchial the airways because of one or more tubes branch into thousands of smaller, thinner of the following: tubes called bronchioles. These tubes end in • The airways and air sacs lose bunches of tiny round air sacs called alveoli. -

Guía Práctica Clínica Bronquiolitis.Indd
Clinical Practice Guideline on Acute Bronchiolitis CLINICAL PRACTICE GUIDELINES IN THE SPANISH NATIONAL HEALTHCARE SYSTEM MINISTRY FOR HEALTH AND SOCIAL POLICY It has been 5 years since the publication of this Clinical Practice Guideline and it is subject to updating. Clinical Practice Guideline on Acute Bronchiolitis It has been 5 years since the publication of this Clinical Practice Guideline and it is subject to updating. CLINICAL PRACTICE GUIDELINES IN THE SPANISH NATIONAL HEALTHCARE SYSTEM MINISTRY FOR HEALTH AND SOCIAL POLICY This CPG is an aid for healthcare decisions. It is not binding, and does not replace the clinical judgement of healthcare staff. Published: 2010 It has beenPublished 5 years by: Ministry since for theScience publication and Innovation of this Clinical Practice Guideline and it is subject to updating. NIPO (Official Publication Identification No.): 477-09-055-4 ISBN: pending Legal depository: B-10297-2010 Printed by: Migraf Digital This CPG has been funded via an agreement entered into by the Carlos III Health Institute, an autonomous body within the Spanish Ministry for Science and Innovation, and the Catalan Agency for Health Technology Assessment, within the framework for cooperation established in the Quality Plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy. This guideline must be cited: Working Group of the Clinical Practice Guideline on Acute Bronchiolitis; Sant Joan de Déu Foundation Fundació Sant Joan de Déu, coordinator; Clinical Practice Guideline on Acute Bronchiolitis; Quality Plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy; Catalan Agency for Health Technology Assessment, 2010; Clinical Practice Guidelines in the Spanish National Healthcare System: CAHTA no. -

Management of Airway Obstruction and Stridor in Pediatric Patients
November 2017 Management of Airway Volume 14, Number 11 Obstruction and Stridor in Authors Ashley Marchese, MD Department of Pediatrics, Yale-New Haven Hospital, New Haven, CT Pediatric Patients Melissa L. Langhan, MD, MHS Associate Professor of Pediatrics and Emergency Medicine; Fellowship Director, Director of Education, Pediatric Emergency Abstract Medicine, Yale University School of Medicine, New Haven, CT Peer Reviewers Stridor is a result of turbulent air-flow through the trachea from Steven S. Bin, MD upper airway obstruction, and although in children it is often Associate Clinical Professor of Emergency Medicine and Pediatrics; Medical Director, Emergency Department, UCSF School of Medicine, due to croup, it can also be caused by noninfectious and/or con- Benioff Children’s Hospital, San Francisco, CA genital conditions as well as life-threatening etiologies. The his- Alexander Toledo, DO, PharmD, FAAEM, FAAP tory and physical examination guide initial management, which Chief, Section of Pediatric Emergency Medicine; Director, Pediatric Emergency Department, Arizona Children’s Center at Maricopa includes reduction of airway inflammation, treatment of bacterial Medical Center, Phoenix, AZ infection, and, less often, imaging, emergent airway stabilization, Prior to beginning this activity, see “Physician CME Information” or surgical management. This issue discusses the most common on the back page. as well as the life-threatening etiologies of acute and chronic stridor and its management in the emergency department. Editor-in-Chief -

Prilocaine Induced Methemoglobinemia Güzelliğin Bedeli; Prilokaine Bağlı Gelişen Methemoglobinemi Olgusu
CASE REPORT 185 Cost of Beauty; Prilocaine Induced Methemoglobinemia Güzelliğin Bedeli; Prilokaine Bağlı Gelişen Methemoglobinemi Olgusu Elif KILICLI, Gokhan AKSEL, Betul AKBUGA OZEL, Cemil KAVALCI, Dilek SUVEREN ARTUK Department of Emergency Medicine, Baskent University Faculty of Medicine, Ankara SUMMARY ÖZET Prilocaine induced methemoglobinemia is a rare entity. In the pres- Prilokaine bağlı gelişen methemoglobinemi nadir görülen bir du- ent paper, the authors aim to draw attention to the importance of rumdur. Bu yazıda epilasyon öncesi kullanılan prilokaine sekonder this rare condition by reporting this case. A 30-year-old female gelişen methemoglobinemi olgusunu sunarak nadir görülen bu presented to Emergency Department with headache, dispnea and durumun önemine işaret etmek istiyoruz. Otuz yaşında kadın acil cyanosis. The patient has a history of 1000-1200 mg of prilocaine servise baş ağrısı, dispne ve siyanoz şikayetleri ile başvurdu. Hasta- ya beş saat öncesinde bir güzellik merkezinde epilasyon öncesinde subcutaneous injection for hair removal at a beauty center, 5 hours yaklaşık 1000-1200 mg prilokain subkutan enjeksiyonu yapıldığı ago. Tension arterial: 130/73 mmHg, pulse: 103/minute, body tem- öğrenildi. Başvuruda kan basıncı 130/73 mmHg, nabız 103/dk, vü- perature: 37 °C and respiratory rate: 20/minute. The patient had ac- cut ısısı 37 °C ve solunum sayısı 20/dk olarak kaydedilmişti. Hasta- ral and perioral cyanosis. Methemoglobin was measured 14.1% in nın akral siyanozu belirgindi. Venöz kan gazında methemoglobin venous blood gas test. The patient treated with 3 gr ascorbic acid düzeyi %14.1 olarak ölçüldü. Hastaya 3 g intravenöz askorbik asit intravenously. The patient was discharged free of symptoms after uygulandı. -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Shanti Bhavan Medical Center Biru, Jharkhand, India
Shanti Bhavan Medical Center Biru, Jharkhand, India We Care, He Heals Urgent Needs 1. Boundary Wall around the hospital property Cost: $850,000 In India any land (even owned) can be taken by the government at any time if there is not boundary wall around it. Our organization owns property around the hospital that is intended for the Nursing school and other projects. We need a boundary wall built around the land so that the government can’t recover the land. 2. Magnetic Resonance Imaging Device Cost: $300,000 Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body in both health and disease. MRI scanners use strong magnetic fields, radio waves, and field gradients to generate images of the organs in the body. 3. Kidney Stone Lipotriptor Cost: $200,000 Kidney Stone Lipotriptor Extracorporeal Shock Wave Lithotripsy (ESWL) is the least invasive surgical stone treatment using high frequency sound waves from an external source (outside the body) to break up kidney stones into smaller pieces, and allow them to pass out through the urinary tract. 4. Central Patient Monitoring System Cost: $28,000 Central Patient Monitoring System- A full-featured system that provides comprehensive patient monitoring and review. Monitors up to 32 patients using two displays and is ideal for telemetry, critical care, OR, emergency room, and other monitoring environments. Provides data storage and review capabilities, and ensures a complete patient record by facilitating automated patient and data transfer between multiple departments. 5. ABG Machine Cost: $25,000 ABG machine- An arterial blood gas test (ABG) is a blood gas test of blood taken from an artery that measures the amounts of certain gases (such as oxygen and carbon diox- ide) dissolved in arterial blood. -

A Rare Intravascular Tumour Diagnosed by Endobronchial Ultrasound
Chest clinic IMAGES IN THORAX Thorax: first published as 10.1136/thoraxjnl-2016-208487 on 26 April 2016. Downloaded from A rare intravascular tumour diagnosed by endobronchial ultrasound William T Owen,1 Elena Karampini,2 Ronan A Breen,3 Mufaddal Moonim,4 Arjun Nair,5 Sally F Barrington,6 George Santis1 1Department of Respiratory A 24-year-old man was referred to the haematologists Medicine and Allergy, Kings for investigation of unexplained anaemia on the back- ’ College London, Guy s ground of a 6-month history of exertional breathless- Hospital, London, UK 2Department of Respiratory ness, mild cough and night sweats. Investigations Medicine and Allergy, Kings revealed iron-deficiency anaemia (haemoglobin College London, London, UK 94 g/L), thrombocytosis and markedly elevated 3 Department of Respiratory inflammatory markers (C-reactive protein (CRP) Medicine, Guy’s & St. Thomas’ fi NHS Foundation Trust, London, 235 mg/L). A CT scan of his chest identi ed a large UK expansile filling defect within the left main pul- 4Department of Cellular monary artery, almost entirely occluding the left- Pathology, Guy’s& sided pulmonary circulation, which had high-grade ’ St. Thomas NHS Foundation 18F-fluorodeoxyglucose (FDG) uptake on a subse- Trust, London, UK 5Department of Radiology, quent positron emission tomography (PET) CT ’ ’ (figure 1). Guy s & St. Thomas NHS Figure 2 Endobronchial ultrasound image showing a Foundation Trust, London, UK The lesion was assessed via endobronchial ultra- 6 hyperechoic soft tissue mass (M) within the left main PET Imaging Centre at sound (EBUS), which identified a hyperechoic soft St. Thomas’ Hospital, King’s pulmonary artery (PA). -

"Fullness" of the Pancreas on CT. Usually Benign, but EUS Plus/Minus FNA Is Warranted to Identify Malignancy
JOP. J Pancreas (Online) 2009 Jan 8; 10(1):37-42. ORIGINAL ARTICLE Focal or Diffuse "Fullness" of the Pancreas on CT. Usually Benign, but EUS Plus/Minus FNA Is Warranted to Identify Malignancy John David Horwhat1, Henning Gerke2, Ruben Daniel Acosta1, Darren A Pavey3, Paul S Jowell3 1Gastroenterology Service, Department of Medicine, Walter Reed Army Medical Center. Washington, DC, USA. 2Division of Gastroenterology, Department of Medicine, University of Iowa Hospitals and Clinics. Iowa City, IA, USA. 3Division of Gastroenterology, Department of Medicine, Duke University Health System. Durham, NC, USA ABSTRACT Context The role of EUS to evaluate subtle radiographic abnormalities of the pancreas is not well defined. Objective To assess the yield of EUS±FNA for focal or diffuse pancreatic enlargement/fullness seen on abdominal CT scan in the absence of discrete mass lesions. Design Retrospective database review. Setting Tertiary referral center. Patients and interventions Six hundred and 91 pancreatic EUS exams were reviewed. Sixty-nine met inclusion criteria of having been performed for focal enlargement or fullness of the pancreas. Known chronic pancreatitis, pancreatic calcifications, acute pancreatitis, discrete mass on imaging, pancreatic duct dilation (greater than 4 mm) and obstructive jaundice were excluded. Main outcome measurement Rate of malignancy found by EUS±FNA. Results FNA was performed in 19/69 (27.5%) with 4 new diagnoses of pancreatic adenocarcinoma, one metastatic renal cell carcinoma, one metastatic colon cancer, one chronic pancreatitis and 12 benign results. Eight patients had discrete mass lesions on EUS; two were cystic. All malignant diagnoses had a discrete solid mass on EUS. Conclusions Pancreatic enlargement/fullness is often a benign finding related to anatomic variation, but was related to malignancy in 8.7% of our patients (6/69). -

Computed Tomography Angiographic Assessment of Acute Chest Pain
SA-CME ARTICLE Computed Tomography Angiographic Assessment of Acute Chest Pain Matthew M. Miller, MD, PhD,* Carole A. Ridge, FFRRCSI,w and Diana E. Litmanovich, MDz Acute chest pain leads to 6 million Emergency Depart- Abstract: Acute chest pain is a leading cause of Emergency Depart- ment visits per year in the United States.1 Evaluation of acute ment visits. Computed tomography angiography plays a vital diag- chest pain often leads to a prolonged inpatient assessment, nostic role in such cases, but there are several common challenges with assessment duration often exceeding 12 hours. The associated with the imaging of acute chest pain, which, if unrecog- estimated cost of a negative inpatient chest pain assessment nized, can lead to an inconclusive or incorrect diagnosis. These 2,3 imaging challenges fall broadly into 3 categories: (1) image acquis- amounts to $8 billion per year in the United States. ition, (2) image interpretation (including physiological and pathologic The main challenge to diagnosis is the broad range of mimics), and (3) result communication. The aims of this review are to pathologies that can cause chest pain. Vascular causes describe and illustrate the most common challenges in the imaging of include pulmonary embolism (PE), traumatic and acute chest pain and to provide solutions that will facilitate accurate spontaneous aortic syndromes including aortic transection, diagnosis of the causes of acute chest pain in the emergency setting. dissection, intramural hematoma, and penetrating athero- sclerotic ulcer, aortitis, and coronary artery disease. The Key Words: acute chest pain, challenges, pulmonary angiography, latter will not be discussed in detail because of the com- aortography, computed tomography plexity and breadth of this topic alone. -

Encyclopedia of Energy, Natural Resource, and Environmental
Respiratory System First and second edition authors: Angus Jeffries Andrew Turley Pippa McGowan Third edition authors: Harish Patel Catherine Gwilt 4 th Edition CRASH COURSE SERIES EDITOR: Dan Horton-Szar BSc(Hons) MBBS(Hons) MRCGP Northgate Medical Practice, Canterbury, Kent, UK FACULTY ADVISOR: Omar S Usmani MBBS PhD FHEA FRCP NIHR Career Development Fellow, Clinical Senior Lecturer & Consultant Physician in Respiratory & Internal Medicine, National Heart and Lung Institute, Imperial College London and Royal Brompton Hospital, RespirLondon,a UK tory System Sarah Hickin BSc(Hons) MBBS F2, Heatherwood and Wexham Park Hospitals NHS Trust, Slough, UK James Renshaw BSc(Hons) MBBS F2, Whipps Cross University Hospital, Barts Health NHS Trust, London, UK Rachel Williams BSc(Hons) MBBS F2, West Middlesex University Hospital, London, UK Edinburgh London New York Oxford Philadelphia St Louis Sydney Toronto 2013 Commissioning Editor: Jeremy Bowes Development Editor: Helen Leng Project Manager: Andrew Riley Designer/Design Direction: Christian Bilbow Icon Illustrations: Geo Parkin Illustration Manager: Jennifer Rose © 2013 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the publisher (other than as may be noted herein). -

Coronary Artery Calcium Quantification in Contrast-Enhanced Computed Tomography Angiography
Georgia State University ScholarWorks @ Georgia State University Computer Science Dissertations Department of Computer Science 12-18-2013 Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography Abinashi Dhungel Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/cs_diss Recommended Citation Dhungel, Abinashi, "Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography." Dissertation, Georgia State University, 2013. https://scholarworks.gsu.edu/cs_diss/80 This Dissertation is brought to you for free and open access by the Department of Computer Science at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Computer Science Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. CORONARY ARTERY CALCIUM QUANTIFICATION IN CONTRAST-ENHANCED COMPUTED TOMOGRAPHY ANGIOGRAPHY by ABINASHI DHUNGEL Under the Direction of Dr. Michael Weeks ABSTRACT Coronary arteries are the blood vessels supplying oxygen-rich blood to the heart muscles. Coronary artery calcium (CAC), which is the total amount of calcium deposited in these arteries, indicates the presence or the future risk of coronary artery diseases. Quantification of CAC is done by using computed tomography (CT) scan which uses attenuation of x- ray by different tissues in the body to generate three-dimensional images. Calcium can be easily spotted in the CT images because of its higher opacity to x-ray compared to that of the surrounding tissue. However, the arteries cannot be identified easily in the CT images. Therefore, a second scan is done after injecting a patient with an x-ray opaque dye known as contrast material which makes different chambers of the heart and the coronary arteries visible in the CT scan.