Morphologically Low-Grade Spiradenocarcinoma: A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malignant Eccrine Adenoma with Sarcomatous (Heterologous) Components: Report of a Rare Skin Adnexal Neoplasm with Literature Review
Open Access Case Report DOI: 10.7759/cureus.12390 Malignant Eccrine Adenoma With Sarcomatous (Heterologous) Components: Report of a Rare Skin Adnexal Neoplasm With Literature Review Hira Ishtiaq 1 , Muhammad Abdulwaasey 1 , Muhammad Usman Tariq 1 , Saira Fatima 2 1. Histopathology, Pathology and Laboratory Medicine, Aga Khan University Hospital, Karachi, PAK 2. Histopathology, Aga Khan University Hospital, Karachi, PAK Corresponding author: Muhammad Usman Tariq, [email protected] Abstract Malignant eccrine spiradenoma (MES) is an exceedingly rare skin adnexal tumor that arises from pre- existing benign eccrine spiradenoma (BES). MES tumors show a wide spectrum of morphological features, posing a diagnostic challenge to the pathologist. Sarcomatous (heterologous) elements are seen in a few of these tumors, further complicating the morphological picture. We herein describe a case of a 66-year-old male who presented with a recently enlarging, ulcerated, nodular skin lesion over the right leg that had been present for the last 25 years. The patient underwent wide local excision of the tumor. Microscopic examination revealed a neoplastic lesion comprising benign and malignant components. The carcinomatous component showed features of infiltrating adenocarcinoma, not otherwise specified, whereas the sarcomatous component showed predominant osteosarcomatous and focal chondrosarcomatous differentiation. The benign component showed morphological and immunohistochemical features of BES. No adjuvant treatment was administered. The patient was alive and disease-free for 14 months, after which he was lost to follow-up. Careful identification and knowledge related to histological diversity are keys to the correct diagnosis of this rare tumor. MESs are potentially aggressive tumors, and therefore, close long-term follow-up should be maintained. -

An Aggressive Treatment for Aggressive Digital Papillary Adenocarcinoma
Continuing Medical Education An Aggressive Treatment for Aggressive Digital Papillary Adenocarcinoma H. Serhat Inaloz, MD, MSc; G.K. Patel, MRCP; Arthur G. Knight, MD, FRCP GOAL To recognize the clinical and histologic signs of aggressive digital papillary adenoma (ADPA) and adenocarcinoma (ADPAca) OBJECTIVES Upon completion of this activity, dermatologists and general practitioners should be able to: 1. Recognize the symptoms of ADPA and ADPAca. 2. Differentiate ADPA from ADPAca. 3. Discuss the immunocytochemistry of ADPA and ADPAca. CME Test on page 210. This article has been peer reviewed and Medicine is accredited by the ACCME to provide approved by Michael Fisher, MD, Professor of continuing medical education for physicians. Medicine, Albert Einstein College of Medicine. Albert Einstein College of Medicine designates Review date: February 2002. this educational activity for a maximum of 1.0 hour This activity has been planned and implemented in category 1 credit toward the AMA Physician’s in accordance with the Essential Areas and Policies Recognition Award. Each physician should claim of the Accreditation Council for Continuing Medical only those hours of credit that he/she actually spent Education through the joint sponsorship of Albert in the educational activity. Einstein College of Medicine and Quadrant This activity has been planned and produced in HealthCom, Inc. The Albert Einstein College of accordance with ACCME Essentials. Aggressive digital papillary adenoma (ADPA) and rule out a possible risk of metastatic carcinoma adenocarcinoma (ADPAca) are adnexal tumors of the skin. Recognition of these tumors is impor- that are not often recognized because of their tant because of a potential risk of local recurrence rarity. -

An Institutional Experience
Original Research Article Skin Adnexal Tumors- An Institutional Experience 1 2* 3 4 5 6 Rekha M Haravi , Roopa K N , Priya Patil , Rujuta Datar , Meena N Jadhav , Shreekant K Kittur 1,5Associate Professor, 2Post Graduate Student, 3,4Assistant Professor, 6Professor & HOD, Department of Pathology, Belgaum Institute of Medical Sciences Dr B R Ambedkar Road, Belagavi, Karnataka – 590001, INDIA. Email: [email protected] Abstract Background: Skin adnexal tumors are a wide spectrum of benign and malignant tumors that differentiate towards one or more adnexal structures found in normal skin. The adnexal structures of skin are the hair follicles, sebaceous glands, eccrine and apocrine sweat glands. These skin adnexal tumors are often difficult to diagnose clinically. This retrospective study was undertaken to know the various histomorphological patterns of skin adnexal tumors at our institution and to determine the incidence among the genders and age groups along with the site distribution. Materials and methods: A total of 40 specimens received and diagnosed as skin adnexal tumors in the department of Pathology at Belgaum Institute of Medical Sciences, Belagavi for a period of 6 years from January 2014 to December 2019 were taken for the study. Histopathological slides prepared from tissue blocks retrieved from departmental archives were reviewed and classified according to the WHO classification 2017. Results: Out of the total 40 samples, benign tumors were 36 (90%) and malignant were 4 (10%). Largest group was the benign tumors of apocrine and eccrine differentiation (47.5%) followed by benign tumors of hair follicle differentiation (40%). Malignant tumors of sebaceous differentiation were 5%, malignant tumors of eccrine and apocrine differentiation were 2.5% and malignant hair follicle differentiation tumors were 2.5% of the total. -

Inherited Skin Tumour Syndromes
CME GENETICS Clinical Medicine 2017 Vol 17, No 6: 562–7 I n h e r i t e d s k i n t u m o u r s y n d r o m e s A u t h o r s : S a r a h B r o w n , A P a u l B r e n n a n B a n d N e i l R a j a n C This article provides an overview of selected genetic skin con- and upper trunk. 1,2 These lesions are fibrofolliculomas, ditions where multiple inherited cutaneous tumours are a cen- trichodiscomas and acrochordons. Patients are also susceptible tral feature. Skin tumours that arise from skin structures such to the development of renal cell carcinoma, lung cysts and as hair, sweat glands and sebaceous glands are called skin pneumothoraces. 3 appendage tumours. These tumours are uncommon, but can Fibrofolliculomas and trichodiscomas clinically present as ABSTRACT have important implications for patient care. Certain appenda- skin/yellow-white coloured dome shaped papules 2–4 mm in geal tumours, particularly when multiple lesions are seen, may diameter (Fig 1 a and Fig 1 b). 4 These lesions usually develop indicate an underlying genetic condition. These tumours may in the third or fourth decade.4 In the case of fibrofolliculoma, not display clinical features that allow a secure diagnosis to be hair specific differentiation is seen, whereas in the case of made, necessitating biopsy and dermatopathological assess- trichodiscoma, differentiation is to the mesodermal component ment. -

The Best Diagnosis Is: H&E, Original Magnification 2
Dermatopathology Diagnosis The best diagnosis is: H&E, original magnification 2. a. adenoid cysticcopy carcinoma arising within a spiradenoma b. cylindroma and spiradenoma collision tumor c. microcysticnot change within a spiradenoma d. mucinous carcinoma arising within a spiradenoma Doe. trichoepithelioma and spiradenoma collision tumor CUTIS H&E, original magnification 100. PLEASE TURN TO PAGE 211 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Amanda F. Marsch, MD; Jeffrey B. Shackelton, MD; Dirk M. Elston, MD Dr. Marsch is from the Department of Dermatology, University of Illinois at Chicago. Drs. Shackelton and Elston are from the Ackerman Academy of Dermatopathology, New York, New York. The authors report no conflict of interest. Correspondence: Amanda F. Marsch, MD, University of Illinois at Chicago, 808 S Wood St, Chicago, IL 60612 ([email protected]). 192 CUTIS® WWW.CUTIS.COM Copyright Cutis 2015. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Trichoepithelioma and Spiradenoma Collision Tumor he coexistence of more than one cutaneous adnexal neoplasm in a single biopsy specimen Tis unusual and is most frequently recognized in the context of a nevus sebaceous or Brooke-Spiegler syndrome, an autosomal-dominant inherited disease characterized by cutaneous adnexal neoplasms, most commonly cylindromas and trichoepitheliomas.1-3 Brooke-Spiegler syndrome is caused by germline muta- tions in the cylindromatosis gene, CYLD, located on band 16q12; it functions as a tumor suppressor gene and has regulatory roles in development, immunity, and inflammation.1 Weyers et al3 first recognized the tendency for adnexal collision tumors to present in patients with Brooke-Spiegler syndrome; they reported a patient with Brooke-Spiegler syndrome with spirad- Figure 1. -

Rippled-Pattern Sebaceoma: a Report of a Lesion on the Back with a Review of the Literature
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Fukui Repository Rippled-pattern sebaceoma: A report of a lesion on the back with a review of the literature Takahiro Kiyohara, M.D., Masanobu Kumakiri, M.D., Hiroaki Kuwahara, M.D., Atsuko Saitoh, M.D., and Shinichi Ansai, M.D. Department of Dermatology (T.K., M.K.), University of Fukui, Fukui; Division of Plastic Surgery (H.K.), Obihiro-Kousei General Hospital, Obihiro: Sapporo Institute for Dermatopathology (S.A.), Sapporo, Japan Address correspondence and reprint requests to: Takahiro Kiyohara, M.D. Department of Dermatology, University of Fukui 23-3 Shimoaizuki, Matsuoka-cho, Yoshida-gun, Fukui 910-1193, Japan Tel: +81 776 61 3111 Fax: +81 776 61 8112 e-mail: kiyo @ fmsrsa.fukui-med.ac.jp Abstract A 68-year-old Japanese man presented with a tumor that had been present for 5 to 6 years on the right back. Physical examination revealed a dome-shaped, 12x13-mm, dark red tumor. The tumor was excised with a 2 to 3-mm margin. The patient has remained free of disease during 77-months of follow-up. Microscopic examination revealed a bulb-like tumor in the dermis, contiguous with the overlying epidermis. It was composed of small, monomorphous, cigar-shaped basaloid cells in linear, parallel rows, resembling the palisading of nuclei of Verocay bodies, and presenting a rippled-pattern. There were scattered cells showing sebaceous differentiation with vacuolated cytoplasm and scalloped nuclei. There were tiny, duct-like spaces. The tumor revealed characteristics of rippled-pattern sebaceoma. -

Genetics of Skin Appendage Neoplasms and Related Syndromes
811 J Med Genet: first published as 10.1136/jmg.2004.025577 on 4 November 2005. Downloaded from REVIEW Genetics of skin appendage neoplasms and related syndromes D A Lee, M E Grossman, P Schneiderman, J T Celebi ............................................................................................................................... J Med Genet 2005;42:811–819. doi: 10.1136/jmg.2004.025577 In the past decade the molecular basis of many inherited tumours in various organ systems such as the breast, thyroid, and endometrium.2 syndromes has been unravelled. This article reviews the clinical and genetic aspects of inherited syndromes that are Clinical features of Cowden syndrome characterised by skin appendage neoplasms, including The cutaneous findings of Cowden syndrome Cowden syndrome, Birt–Hogg–Dube syndrome, naevoid include trichilemmomas, oral papillomas, and acral and palmoplantar keratoses. The cutaneous basal cell carcinoma syndrome, generalised basaloid hallmark of the disease is multiple trichilemmo- follicular hamartoma syndrome, Bazex syndrome, Brooke– mas which present clinically as rough hyperker- Spiegler syndrome, familial cylindromatosis, multiple atotic papules typically localised on the face (nasolabial folds, nose, upper lip, forehead, ears3 familial trichoepitheliomas, and Muir–Torre syndrome. (fig 1A, 1C, 1D). Trichilemmomas are benign ........................................................................... skin appendage tumours or hamartomas that show differentiation towards the hair follicles kin consists of both epidermal and dermal (specifically for the infundibulum of the hair 4 components. The epidermis is a stratified follicle). Oral papillomas clinically give the lips, Ssquamous epithelium that rests on top of a gingiva, and tongue a ‘‘cobblestone’’ appearance basement membrane, which separates it and its and histopathologically show features of 3 appendages from the underlying mesenchymally fibroma. The mucocutaneous manifestations of derived dermis. -

Malignant Cylindroma in a Patient with Brooke-Spiegler Syndrome
DERMATOLOGY PRACTICAL & CONCEPTUAL www.derm101.com Malignant cylindroma in a patient with Brooke-Spiegler syndrome Liliane Borik1,2, Patricia Heller2, Monica Shrivastava3, Viktoryia Kazlouskaya2 1 Division of Immunology, Allergy and Infectious Diseases, Department of Dermatology, Medical University of Vienna, Vienna, Austria 2 Ackerman Academy of Dermatopathology, New York, NY, USA 3 Advanced Laser Skin Center, Teaneck, NJ, USA Key words: adnexal neoplasm, apocrine neoplasm, cylindroma, cylindrocarcinoma Citation: Borik L, Heller P, Shrivastava M, Kazlouskaya V. Malignant cylindroma in a patient with Brooke-Spiegler syndrome. Dermatol Pract Concept 2015;5(2):9. doi: 10.5826/dpc.0502a09 Received: October 17, 2014; Accepted: January 9, 2015; Published: April 30, 2015 Copyright: ©2015 Kazlouskaya et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: None. Competing interests: The authors have no conflicts of interest to disclose. All authors have contributed significantly to this publication. Corresponding author: Viktoryia Kazlouskaya, MD, PhD, Ackerman Academy of Dermatopathology ,145 E 32 St, 10th Fl, New York, NY 10036, USA. Tel. 347-488-8058. E-mail: [email protected] ABSTRACT Malignant cylindroma (cylindromatous carcinoma, cylindrocarcinoma) is the malignant counterpart of benign cylindroma. It is a rare neoplasm, more often developing in the setting of multiple pre- existing benign neoplasms. Herein we present an additional case of malignant transformation of the cylindroma diagnosed in an 83-year-old patient with known Brooke-Spiegler syndrome. Case presentation Figure 1. Multiple nodules An 83-year-old patient presented to the dermatologist in the central numerous times with multiple lesions on the face, scalp and face. -

Rotana Alsaggaf, MS
Neoplasms and Factors Associated with Their Development in Patients Diagnosed with Myotonic Dystrophy Type I Item Type dissertation Authors Alsaggaf, Rotana Publication Date 2018 Abstract Background. Recent epidemiological studies have provided evidence that myotonic dystrophy type I (DM1) patients are at excess risk of cancer, but inconsistencies in reported cancer sites exist. The risk of benign tumors and contributing factors to tu... Keywords Cancer; Tumors; Cataract; Comorbidity; Diabetes Mellitus; Myotonic Dystrophy; Neoplasms; Thyroid Diseases Download date 07/10/2021 07:06:48 Link to Item http://hdl.handle.net/10713/7926 Rotana Alsaggaf, M.S. Pre-doctoral Fellow - Clinical Genetics Branch, Division of Cancer Epidemiology & Genetics, National Cancer Institute, NIH PhD Candidate – Department of Epidemiology & Public Health, University of Maryland, Baltimore Contact Information Business Address 9609 Medical Center Drive, 6E530 Rockville, MD 20850 Business Phone 240-276-6402 Emails [email protected] [email protected] Education University of Maryland – Baltimore, Baltimore, MD Ongoing Ph.D. Epidemiology Expected graduation: May 2018 2015 M.S. Epidemiology & Preventive Medicine Concentration: Human Genetics 2014 GradCert. Research Ethics Colorado State University, Fort Collins, CO 2009 B.S. Biological Science Minor: Biomedical Sciences 2009 Cert. Biomedical Engineering Interdisciplinary studies program Professional Experience Research Experience 2016 – present Pre-doctoral Fellow National Cancer Institute, National Institutes -

12 IJBMRF20131339 Dr Sindhoori Komma
Int J Biol Med Res. 2013; 4(4): 3707-3709 Int J Biol Med Res www.biomedscidirect.com Volume 3, Issue 1, Jan 2012 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research BioMedSciDirect Journal homepage: www.biomedscidirect.com International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Case Report An Interesting Case Of Proliferating Trichilemmal Cysts And Lipoma Of The Scalp Sindhoori .K*a, Kishore Kumar B.N b, Prem Sai Reddy.B c, Sreeramulu.P Nd, Udaya Kumar.Me. aPost graduate department of Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.pin code-563101 bProf and HOD, Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 cPost graduate, Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 dProf and unit chief, Surgery, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 eProfessor, Pathology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 A R T I C L E I N F O A B S T R A C T Keywords: A 60 year old male patient presented with multiple slow growing lesions on scalp for past Trichilemmal cysts 25years. Patient was evaluated with radiographs and computed tomography (CT). Patient Radiographs underwent simple excision of the lesions and the diagnosis was confirmed on histopathology as Computed tomography proliferating trichilemmal cysts and lipoma of the scalp. KEY WORDS: Lipoma CTscan. c Copyright 2010 BioMedSciDirect Publications IJBMR -ISSN: 0976:6685. All rights reserved. 1. Introduction Proliferating trichilemmal cysts(PTCs) also known as pilar Figure 1- photograph of patients scalp with multiple soft cyst is a benign adnexal tumor of skin, related to the isthmus of the tissue swelling hair follicle1. -
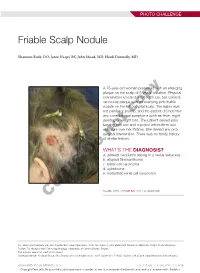
Friable Scalp Nodule
PHOTO CHALLENGE Friable Scalp Nodule Shannon Buck, DO; Jaree Naqvi, BS; John Moad, MD; Heidi Donnelly, MD A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years’ duration. Physical examination revealed a 5.6×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic,copy and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgicalnot intervention. There was no family history of similar lesions. WHAT’S THE DIAGNOSIS? Doa. adnexal neoplasm arising in a nevus sebaceus b. atypical fibroxanthoma c. basal cell carcinoma d. cylindroma e. metastatic renal cell carcinoma CUTIS PLEASE TURN TO PAGE E20 FOR THE DIAGNOSIS Drs. Buck and Donnelly are from Dayton Skin Care Specialists, Ohio. Mr. Naqvi is from Boonshoft School of Medicine, Wright State University, Dayton. Dr. Moad is from Dermatopathology Laboratory of Central States, Dayton. The authors report no conflict of interest. Correspondence: Shannon Buck, DO, Dayton Skin Care Specialists, 3025 Governor’s Pl Blvd, Dayton, OH 45409 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 105 NO. 1 I JANUARY 2020 E19 Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Adnexal Neoplasm Arising in a Nevus Sebaceus iopsy of the lesion showed a proliferation of basa- secondary neoplasms, 88% of which were benign.2 The loid-appearing cells with focal ductal differentiation origins of these neoplasms can be epithelial, sebaceous, Band ulceration consistent with poroma (Figure 1). -

Dermal Cylindroma of the Scalp (Turban Tumour) and Subjacent Calvarian Defects
ANTICANCER RESEARCH 30: 1793-1798 (2010) Dermal Cylindroma of the Scalp (Turban Tumour) and Subjacent Calvarian Defects REINHARD E. FRIEDRICH Department of Oral and Maxillofacial Surgery, Eppendorf University Hospital, University of Hamburg, Germany Abstract. This case report describes the diagnosis and Case Report therapy of a patient with a 40-year history of multiple potato-like tumours growing in the head and neck region. Patient. A seventy-five-year-old woman was referred to the The tumour proved to be a cylindroma, associated with Department of Oral and Maxillofacial Surgery from the calvarian defects. Further facial tumours were diagnosed as Department of Dermatology, Eppendorf University Hospital, trichoepitheliomas. This association of findings was for treatment of an extensive cylindroma of the entire scalp. pathognomonic for Brooke-Spiegler’s syndrome. Complete The patient first sought for medical advice in the University work-up of the resection specimen excluded any malignant Hospital 4 years previously but had refused any surgical transformation of the tumour. A long lasting history of treatment (Figure 1). On presentation, the patient was in cylindroma and evidence for bone destruction, not associated generally good condition. The primary reason the patient with a malignant transformation, is extremely rare in decided to seek surgical treatment was an ill-fit of her wig Brooke-Spiegler’s syndrome. (Figure 2). The patient’s medical history showed an early onset of the enlargement of the skin of her skull and the development Cylindroma is a slow growing, benign tumour of the skin (1), of potato-like tumours about 40 years earlier. At the same time, affecting preferentially the head and neck region, especially she noticed a growing baldness in the area of tumour growth the capillitium and forehead (2, 3).