An Institutional Experience
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

第32回日本皮膚病理組織学会学術大会 診断投票結果 口演 1 Drug Eruption 13, うち Erythema Multif
第32回日本皮膚病理組織学会学術大会 診断投票結果 口演 1 Drug eruption 13, うち erythema multiforme 1, Interface dermatitis 1, GVHD type 1 Cutaneous reaction due to CCR4 3, うち Dysplastic epidermal hyperplasia 2, Adverse reaction 1 Erythema multiforme 3 PLEVA 1 Vacuolar type interface dermatitis 1 口演 2 Syringofibroadenoma 15, うち + amyloid 1 Syringofibroadenoma with BCC 5 Basal cell carcinoma 4, うち Pinkus type of BCC with syringofibroadenoma 2 口演 3 Darier disease 5 Hailey-Hailey disease 4 Pemphigus 3, うち Pemphigus Vegetans 1, Neonatal pemphigus 1 Grover's disease 4 Epidermal nevus 5, うち Acantholytic (dyskeratotic) epidermal nevus 4, Linear epidermal nevus 1 口演 4 Hydradenoma 13, うち Clear cell hidradenoma 12, Nodular hidradenoma 1 Sebaceous adenoma 1 Trichilemmoma 1 Metastatic tumor 8, うち ~ renal carcinoma6, ~ Clear cell carcinoma 2 口演 5 Apocrine carcinoma 3, うち ~with pagetoid spreading 2 Ectopic breast carcinoma(invasive ductal type)with pagetoid phenomenon 2 Extramammary Paget's disease 12, うち Paget carcinoma 3, ~ with Apocrine adenoma 2, ~ with Tubular adenoma 1, Invasive ~ 1, +Skin metastasis 1, With syringoma 1, with Microcystic Adnexal Carcinoma 1 Syringomatous carcinoma 2, うち ~with paget phenomenon 1 Tubular adenocartinoma 1 Tubular (apocrine) adenoma 2 Syringoma 1 口演 6 Dermatofibroma 10, うち Lipidized ~ 3, Hemosiderotic deep cellular ~ 2, Xanthomatous ~ 1, ~ Histiocytoid variant 1 Fibous histiocytoma 8, うち Atypical ~ 3, Malignant ~ 2, Aneurismal ~ 2 Undifferentiated pleomorphic sarcoma 2 Progressive nodular histiocytosis 1 Squamous -

Pdf 344.65 K
Differential Diagnosis of Basal Cell Carcinoma and Benign Tumors of Hair Follicles Using CD34 RESEARCH COMMUNICATION Differential Diagnosis of Basal Cell Carcinoma and Benign Tumors of Cutaneous Appendages Originating from Hair Follicles by Using CD34 Demet Sengul1, Ilker Sengul2*, Muzeyyen Hesna Astarci3, Huseyin Ustun3, Gamze Mocan4 Abstract Background and Aims: Differential diagnosis of the group of benign trichoblastomas, trichofolliculomas, trichoadenomas and trichoepitheliomas, and basal cell carcinomas (BCCs) is troublesome for the clinician as well as the pathologist, especially when only small biopsy specimens are available. Here we investigated whether CD34 expression might be of assistance. Methods: Thirty benign tumors of cutaneous appendages originating from hair follicles (BTCOHF) and 30 BCCs were retrieved from our archives and immunohistochemically stained. CD 34 expression was graded from [0] to [2+] and compared among the groups and subgroups. Results: There was no significant difference between the degree of expression between [0] and [1+] and [0] and [2+] for each group. However, [1+] and [2+] immunopositivity of BTCOHFs was significantly stronger than in BCCs (p= 0.014). Conclusions: CD34 may contribute to differential diagnosis of skin lesions. Keywords: Basal cell cancer - hair follicle lesions - CD 34 immunohistochemistry Asian Pacific J Cancer Prev, 11, 1615-1619 Introduction in 1958. TAs occur as a nodular lesion usually on the face and buttocks (Rahbari et al., 1977, Swaroop et al., 2008) Ackerman et al classified benign tumors of cutaneous and have a variant of verrucous TA mimicing seboreic appendages originating from hair follicle (BTCOHF)’s keratosis. using eight textbooks of dermatopathology in 2001 as Trichoepithelioma (TE) is a benign skin tumor with germ tumors of hair follicle and hamartomas, infindubular follicular differentiation determined in the classification and isthmic tumors, tumors of external layer, tumors of WHO as the synonym of TB (Cotton, 1991). -

Inherited Skin Tumour Syndromes
CME GENETICS Clinical Medicine 2017 Vol 17, No 6: 562–7 I n h e r i t e d s k i n t u m o u r s y n d r o m e s A u t h o r s : S a r a h B r o w n , A P a u l B r e n n a n B a n d N e i l R a j a n C This article provides an overview of selected genetic skin con- and upper trunk. 1,2 These lesions are fibrofolliculomas, ditions where multiple inherited cutaneous tumours are a cen- trichodiscomas and acrochordons. Patients are also susceptible tral feature. Skin tumours that arise from skin structures such to the development of renal cell carcinoma, lung cysts and as hair, sweat glands and sebaceous glands are called skin pneumothoraces. 3 appendage tumours. These tumours are uncommon, but can Fibrofolliculomas and trichodiscomas clinically present as ABSTRACT have important implications for patient care. Certain appenda- skin/yellow-white coloured dome shaped papules 2–4 mm in geal tumours, particularly when multiple lesions are seen, may diameter (Fig 1 a and Fig 1 b). 4 These lesions usually develop indicate an underlying genetic condition. These tumours may in the third or fourth decade.4 In the case of fibrofolliculoma, not display clinical features that allow a secure diagnosis to be hair specific differentiation is seen, whereas in the case of made, necessitating biopsy and dermatopathological assess- trichodiscoma, differentiation is to the mesodermal component ment. -

The Best Diagnosis Is: H&E, Original Magnification 2
Dermatopathology Diagnosis The best diagnosis is: H&E, original magnification 2. a. adenoid cysticcopy carcinoma arising within a spiradenoma b. cylindroma and spiradenoma collision tumor c. microcysticnot change within a spiradenoma d. mucinous carcinoma arising within a spiradenoma Doe. trichoepithelioma and spiradenoma collision tumor CUTIS H&E, original magnification 100. PLEASE TURN TO PAGE 211 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Amanda F. Marsch, MD; Jeffrey B. Shackelton, MD; Dirk M. Elston, MD Dr. Marsch is from the Department of Dermatology, University of Illinois at Chicago. Drs. Shackelton and Elston are from the Ackerman Academy of Dermatopathology, New York, New York. The authors report no conflict of interest. Correspondence: Amanda F. Marsch, MD, University of Illinois at Chicago, 808 S Wood St, Chicago, IL 60612 ([email protected]). 192 CUTIS® WWW.CUTIS.COM Copyright Cutis 2015. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Trichoepithelioma and Spiradenoma Collision Tumor he coexistence of more than one cutaneous adnexal neoplasm in a single biopsy specimen Tis unusual and is most frequently recognized in the context of a nevus sebaceous or Brooke-Spiegler syndrome, an autosomal-dominant inherited disease characterized by cutaneous adnexal neoplasms, most commonly cylindromas and trichoepitheliomas.1-3 Brooke-Spiegler syndrome is caused by germline muta- tions in the cylindromatosis gene, CYLD, located on band 16q12; it functions as a tumor suppressor gene and has regulatory roles in development, immunity, and inflammation.1 Weyers et al3 first recognized the tendency for adnexal collision tumors to present in patients with Brooke-Spiegler syndrome; they reported a patient with Brooke-Spiegler syndrome with spirad- Figure 1. -

Rippled-Pattern Sebaceoma: a Report of a Lesion on the Back with a Review of the Literature
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Fukui Repository Rippled-pattern sebaceoma: A report of a lesion on the back with a review of the literature Takahiro Kiyohara, M.D., Masanobu Kumakiri, M.D., Hiroaki Kuwahara, M.D., Atsuko Saitoh, M.D., and Shinichi Ansai, M.D. Department of Dermatology (T.K., M.K.), University of Fukui, Fukui; Division of Plastic Surgery (H.K.), Obihiro-Kousei General Hospital, Obihiro: Sapporo Institute for Dermatopathology (S.A.), Sapporo, Japan Address correspondence and reprint requests to: Takahiro Kiyohara, M.D. Department of Dermatology, University of Fukui 23-3 Shimoaizuki, Matsuoka-cho, Yoshida-gun, Fukui 910-1193, Japan Tel: +81 776 61 3111 Fax: +81 776 61 8112 e-mail: kiyo @ fmsrsa.fukui-med.ac.jp Abstract A 68-year-old Japanese man presented with a tumor that had been present for 5 to 6 years on the right back. Physical examination revealed a dome-shaped, 12x13-mm, dark red tumor. The tumor was excised with a 2 to 3-mm margin. The patient has remained free of disease during 77-months of follow-up. Microscopic examination revealed a bulb-like tumor in the dermis, contiguous with the overlying epidermis. It was composed of small, monomorphous, cigar-shaped basaloid cells in linear, parallel rows, resembling the palisading of nuclei of Verocay bodies, and presenting a rippled-pattern. There were scattered cells showing sebaceous differentiation with vacuolated cytoplasm and scalloped nuclei. There were tiny, duct-like spaces. The tumor revealed characteristics of rippled-pattern sebaceoma. -

Nevus Sebaceus with Syringocystadenoma
UC Davis Dermatology Online Journal Title Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions Permalink https://escholarship.org/uc/item/85k968bk Journal Dermatology Online Journal, 26(2) Authors Basu, Pallavi Erickson, Christof P Calame, Antoanella et al. Publication Date 2020 DOI 10.5070/D3262047411 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 26 Number 2| February 2020| Dermatology Online Journal || Case Report 26(2):5 Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions Pallavi Basu1 MPH, Christof P Erickson2 MD, Antoanella Calame2 MD, and Philip R Cohen3,4 MD Affiliations: 1School of Medicine, University of California San Diego, La Jolla, California, USA, 2Compass Dermatopathology, San Diego, California, USA, 3San Diego Family Dermatology, National City, California, USA, 4Touro California College of Osteopathic Medicine, Vallejo, California, USA Corresponding Authors: Pallavi Basu, MPH, 8528 Via Mallorca, Apartment G, La Jolla, CA 92037, Tel: 818-917-1786, Email: [email protected]; Philip R. Cohen MD, 10991 Twinleaf Court, San Diego, CA 92131, Email: [email protected] with nevus sebaceus who not only developed Abstract syringocystadenoma papilliferum but also prurigo Nevus sebaceus is a benign skin hamartoma of nodularis in the inferior portion of her lesion is congenital onset that grows during puberty, and in described. Complete excision of the residual nevus adulthood can develop secondary benign and sebaceus also revealed three concurrent additional malignant neoplasms. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Genetics of Skin Appendage Neoplasms and Related Syndromes
811 J Med Genet: first published as 10.1136/jmg.2004.025577 on 4 November 2005. Downloaded from REVIEW Genetics of skin appendage neoplasms and related syndromes D A Lee, M E Grossman, P Schneiderman, J T Celebi ............................................................................................................................... J Med Genet 2005;42:811–819. doi: 10.1136/jmg.2004.025577 In the past decade the molecular basis of many inherited tumours in various organ systems such as the breast, thyroid, and endometrium.2 syndromes has been unravelled. This article reviews the clinical and genetic aspects of inherited syndromes that are Clinical features of Cowden syndrome characterised by skin appendage neoplasms, including The cutaneous findings of Cowden syndrome Cowden syndrome, Birt–Hogg–Dube syndrome, naevoid include trichilemmomas, oral papillomas, and acral and palmoplantar keratoses. The cutaneous basal cell carcinoma syndrome, generalised basaloid hallmark of the disease is multiple trichilemmo- follicular hamartoma syndrome, Bazex syndrome, Brooke– mas which present clinically as rough hyperker- Spiegler syndrome, familial cylindromatosis, multiple atotic papules typically localised on the face (nasolabial folds, nose, upper lip, forehead, ears3 familial trichoepitheliomas, and Muir–Torre syndrome. (fig 1A, 1C, 1D). Trichilemmomas are benign ........................................................................... skin appendage tumours or hamartomas that show differentiation towards the hair follicles kin consists of both epidermal and dermal (specifically for the infundibulum of the hair 4 components. The epidermis is a stratified follicle). Oral papillomas clinically give the lips, Ssquamous epithelium that rests on top of a gingiva, and tongue a ‘‘cobblestone’’ appearance basement membrane, which separates it and its and histopathologically show features of 3 appendages from the underlying mesenchymally fibroma. The mucocutaneous manifestations of derived dermis. -

Case 12 Female 71. Longstanding Nodule from Shoulder Recently Enlarging
Case 12 Female 71. Longstanding nodule from shoulder recently enlarging. Clinically sebaceous cyst Case 12 Female 71. Longstanding nodule from shoulder recently enlarging. Clinically sebaceous cyst Case 12 Malignant areas with marked atypia Case 12: Malignant spiradenoma (spiradenocarcinoma), poorly differenDated, arising in a benign spiradenoma – a relavely rare occurence Spiradenocarcinoma: spiradenocarcinoma either 1) well differenDated and low grade resembling spiradenoma and retain lobularity, but no lymphocytes or two cell paern, or 2) Poorly differenDated with no obvious spiradenomatous differenDaon & rely on benign counterpart for diagnosis (as in this case) & Shows typical features of a malignant neoplasm: spiradenoma • infiltrave border • lacks organised structure of spiradenoma • Lacks clearly demarcated two cell populaon of spiradnoma Case 12: Malignant spiradenoma (spiradenocarcinoma), poorly differenDated, arising in a benign spiradenoma – a relavely rare occurence spiradenocarcinoma • typical features of a malignant neoplasm: • Larger cells , overlapping nuclei. • Increased mitoDc acDvity and cellular pleomorphism. spiradenoma • Necrosis may occur (but can get degnerave change in spiradenoma mimicking necrosis) • OOen long history, elderly, at any site Spiradenoma Spiradenocarcinoma Case 12: Malignant spiradenoma (spiradenocarcinoma), poorly differenDated, arising in a benign spiradenoma – a relavely rare occurence •72 paents in metaanlysis •35 paents with no distant metastasis, local resecDon resulted in100% disease-free survival. •12 had lymph node metastases but no distant metastases • Of 7 paents with lymph node but no distant metastasis treated with surgical resecDon and lymph node dissecDon, 6 paents remained disease-free at final follow-up evaluaon •24 cases with confirmed distant metastac disease •median survival 16 mpnths • paent survival did not significantly differ between local resecDon and surgery with adjuvant chemoradiotherapy CONCLUSIONS: An aggressive surgical approach is supported in the absence of metastasis. -

12 IJBMRF20131339 Dr Sindhoori Komma
Int J Biol Med Res. 2013; 4(4): 3707-3709 Int J Biol Med Res www.biomedscidirect.com Volume 3, Issue 1, Jan 2012 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research BioMedSciDirect Journal homepage: www.biomedscidirect.com International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Case Report An Interesting Case Of Proliferating Trichilemmal Cysts And Lipoma Of The Scalp Sindhoori .K*a, Kishore Kumar B.N b, Prem Sai Reddy.B c, Sreeramulu.P Nd, Udaya Kumar.Me. aPost graduate department of Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.pin code-563101 bProf and HOD, Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 cPost graduate, Radiology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 dProf and unit chief, Surgery, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 eProfessor, Pathology, Sri Devaraj Urs Medical College, Kolar, Karnataka.563101 A R T I C L E I N F O A B S T R A C T Keywords: A 60 year old male patient presented with multiple slow growing lesions on scalp for past Trichilemmal cysts 25years. Patient was evaluated with radiographs and computed tomography (CT). Patient Radiographs underwent simple excision of the lesions and the diagnosis was confirmed on histopathology as Computed tomography proliferating trichilemmal cysts and lipoma of the scalp. KEY WORDS: Lipoma CTscan. c Copyright 2010 BioMedSciDirect Publications IJBMR -ISSN: 0976:6685. All rights reserved. 1. Introduction Proliferating trichilemmal cysts(PTCs) also known as pilar Figure 1- photograph of patients scalp with multiple soft cyst is a benign adnexal tumor of skin, related to the isthmus of the tissue swelling hair follicle1. -
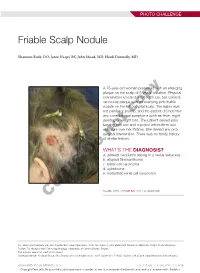
Friable Scalp Nodule
PHOTO CHALLENGE Friable Scalp Nodule Shannon Buck, DO; Jaree Naqvi, BS; John Moad, MD; Heidi Donnelly, MD A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years’ duration. Physical examination revealed a 5.6×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic,copy and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgicalnot intervention. There was no family history of similar lesions. WHAT’S THE DIAGNOSIS? Doa. adnexal neoplasm arising in a nevus sebaceus b. atypical fibroxanthoma c. basal cell carcinoma d. cylindroma e. metastatic renal cell carcinoma CUTIS PLEASE TURN TO PAGE E20 FOR THE DIAGNOSIS Drs. Buck and Donnelly are from Dayton Skin Care Specialists, Ohio. Mr. Naqvi is from Boonshoft School of Medicine, Wright State University, Dayton. Dr. Moad is from Dermatopathology Laboratory of Central States, Dayton. The authors report no conflict of interest. Correspondence: Shannon Buck, DO, Dayton Skin Care Specialists, 3025 Governor’s Pl Blvd, Dayton, OH 45409 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 105 NO. 1 I JANUARY 2020 E19 Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Adnexal Neoplasm Arising in a Nevus Sebaceus iopsy of the lesion showed a proliferation of basa- secondary neoplasms, 88% of which were benign.2 The loid-appearing cells with focal ductal differentiation origins of these neoplasms can be epithelial, sebaceous, Band ulceration consistent with poroma (Figure 1). -

Modpathol20166.Pdf
ANNUAL MEETING ABSTRACTS 125A liver (3), abdominal wall (1) and lung (1). Of the 33 FNA cases, 30 were primary The average DNA concentration for LBC samples was 43.2 ng/µl and FFPE was 12.3 AML (28 in kidney and 2 in liver) and 3 were metastasis (in liver, lung and abdominal ng/µl. Mean average read depths were 10,899 and 7,980 for LBC and FFPE, respectively. wall, respectively). FNA diagnoses included consistent with or favor AML (16, 49%), Five FFPE and 1 LBC specimens had minimum read depths below quality control descriptive (13), non-diagnostic (1) and erroneous diagnosis (3). Of the latter 3 cases standards (<100) involving primarily PIK3CA (6) and BRAF (3). (all in the kidney), two were called “clear cell renal cell carcinoma” due to predominant Targeted genes were concordant across specimen types. For both FFPE and LBC, NGS epithelioid component and one was called “pleomorphic malignancy”. Two renal AMLs detected KRAS mutations previously identified by PCR. In addition, all 20 specimens had co-existing metastatic carcinoma (neuroendocrine carcinoma from pancreas and showed a common EGFR polymorphism c.2361G>A. metastatic lung adenocarcinoma, respectively). Conclusions: NGS results of FFPE and LBC (DNA from cell pellets) were consistent, Upon review, smooth muscle component was most commonly seen (19), followed but the superior coverage depth of LBC specimens supports its clinical use. Our initial by vascular component (17) and adipose tissue (6). Only 4 cases showed all the three experience using NGS on 128 clinical lung cancer FNAs detected KRAS mutations in components; 13 cases had 2 components and 7 cases had smooth muscle component 30 patients and EGFR in 16; qualifying 8 for tyrosine kinase inhibitor therapy.