Chapter-2 Semiconcuctors Diodes & Transistors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schottky Diodes Selection Guide
SCHOTTKY DIODES ( HOT - CARRIER ) pag A 1 Schottky diodes selection guide For HIGH SENSITIVITY , ZERO-BIAS or LOW BARRIER applications --- for lab detectors as RF detector with sweep generator --- RF fields detector, electromagnetic pollution, TAG , etc… --- passive or active mobile phones and bugs detector diode TSS Glass SMD Ceramic or special (tangential sensitivity) case case case HSMS 2850 - 2851 -59 dBm @ 2 GHz SMS 7630 -55 dBm @ 10 GHz these are the much sensitive diodes at usable up to 18 GHz ZERO BIAS -53 dBm @ 2 GHz ND 4991 - 1SS276 DDC2353 -55 dBm @ 6 GHz LOW BARRIER up to 20 GHz from -54 dBm to -52 dBm all BAT 15… types are LOW BARRIER up to 24 GHz depending on type high sensitivity vatious types available -56 dBm @ 2 GHz with bias HP 5082-2824 HSMS.282…series low barrier, up to millimeter freq. beam lead version version with leads of the famous 1N821 point-contact 1N21 - 23 silicon , up to 5 GHz NOTE : high sensitivity silicon or germanium diodes for detectors are available too, see VARIOUS DIODES for : RECEIVING MIXERS - RF DETECTORS - SAMPLING freq. config. glass case SMD or plastic case ceramic case up to 500 MHz BAT 42 - 43 - 46 - 48 - 85 - 86 BAS 40-…- BAT 64-.... 5082.2800 - BAT 45 - 82 - 83 single HSMS 28.... , BAT 68 up to HSCH 1001 2 GHz pair 5082.2804 BAS 70... , HSMS 28... quad 5082.2836 ND 487C1-3R 5082.2810, 2811, 2817 2824, up to HSMS 2810 , 2820 single 2835, 2900, MA4853 ND4991 BAT 17 , BAT 68 3 - 5 1SS154 ,BA 481, QSCH 5374 pair 5082.2826, 2912 HSMS 28…. -

Special Diodes 2113
CHAPTER54 Learning Objectives ➣ Zener Diode SPECIAL ➣ Voltage Regulation ➣ Zener Diode as Peak Clipper DIODES ➣ Meter Protection ➣ Zener Diode as a Reference Element ➣ Tunneling Effect ➣ Tunnel Diode ➣ Tunnel Diode Oscillator ➣ Varactor Diode ➣ PIN Diode ➣ Schottky Diode ➣ Step Recovery Diode ➣ Gunn Diode ➣ IMPATT Diode Ç A major application for zener diodes is voltage regulation in dc power supplies. Zener diode maintains a nearly constant dc voltage under the proper operating conditions. 2112 Electrical Technology 54.1. Zener Diode It is a reverse-biased heavily-doped silicon (or germanium) P-N junction diode which is oper- ated in the breakdown region where current is limited by both external resistance and power dissipa- tion of the diode. Silicon is perferred to Ge because of its higher temperature and current capability. As seen from Art. 52.3, when a diode breaks down, both Zener and avalanche effects are present although usually one or the other predominates depending on the value of reverse voltage. At reverse voltages less than 6 V, Zener effect predominates whereas above 6 V, avalanche effect is predomi- nant. Strictly speaking, the first one should be called Zener diode and the second one as avalanche diode but the general practice is to call both types as Zener diodes. Zener breakdown occurs due to breaking of covalent bonds by the strong electric field set up in the depletion region by the reverse voltage. It produces an extremely large number of electrons and holes which constitute the reverse saturation current (now called Zener current, Iz) whose value is limited only by the external resistance in the circuit. -

Thermionic and Gaseous State Diodes
THERMIONIC AND GASEOUS STATE DIODES Thermionic and gaseous state (vacuum tube) diodes Thermionic diodes are thermionic-valve devices (also known as vacuum tubes, tubes, or valves), which are arrangements of electrodes surrounded by a vacuum within a glass envelope. Early examples were fairly similar in appearance to incandescent light bulbs. In thermionic valve diodes, a current through the heater filament indirectly heats the cathode, another internal electrode treated with a mixture of barium and strontium oxides, which are oxides of alkaline earth metals; these substances are chosen because they have a small work function. (Some valves use direct heating, in which a tungsten filament acts as both heater and cathode.) The heat causes thermionic emission of electrons into the vacuum. In forward operation, a surrounding metal electrode called the anode is positively charged so that it electrostatically attracts the emitted electrons. However, electrons are not easily released from the unheated anode surface when the voltage polarity is reversed. Hence, any reverse flow is negligible. For much of the 20th century, thermionic valve diodes were used in analog signal applications, and as rectifiers in many power supplies. Today, valve diodes are only used in niche applications such as rectifiers in electric guitar and high-end audio amplifiers as well as specialized high-voltage equipment. Semiconductor diodes A modern semiconductor diode is made of a crystal of semiconductor like silicon that has impurities added to it to create a region on one side that contains negative charge carriers (electrons), called n-type semiconductor, and a region on the other side that contains positive charge carriers (holes), called p-type semiconductor. -

CHAPTER 11 HPD (Hybrid Photo-Detector)
CHAPTER 11 HPD (Hybrid Photo-Detector) HPD (Hybrid Photo-Detector) is a completely new photomultiplier tube that incorporates a semiconductor element in an evacuated elec- tron tube. In HPD operation, photoelectrons emitted from the photo- cathode are accelerated to directly strike the semiconductor where their numbers are increased. Features offered by the HPD are extremely little fluctuation during the multiplication, high electron resolution, and excellent stability. © 2007 HAMAMATSU PHOTONICS K. K. 210 CHAPTER 11 HPD (Hybrid Photo-Detector) 11.1 Operating Principle of HPDs As shown in Figure 11-1, an HPD consists of a photocathode for converting light into photoelectrons and a semiconductor element (avalanche diode or AD) which is the target for "electron bombardment" by photo- electrons. The HPD operates on the following principle: when light enters the photocathode, photoelectrons are emitted according to the amount of light; these photoelectrons are accelerated by a high-intensity electric field of a few kilovolts to several dozen kilovolts applied to the photocathode; they are then bombarded onto the target semiconductor where electron-hole pairs are generated according to the incident energy of the photoelectrons. This is called "electron bombardment gain". A typical relation between this electron bom- bardment gain and the photocathode supply voltage is plotted in Figure 11-2. In principle, this electron bom- bardment gain is proportional to the photocathode supply voltage. However, there is actually a loss of energy in the electron bombardment due to the insensitive surface layer of the semiconductor, so their proportional relation does not hold at a low voltage. In Figure 11-2, the voltage at a point on the voltage axis (horizontal axis) where the dotted line intersects is called the threshold voltage [Vth]. -

(Or Varicap) Diode
Radio and Electronics Cookbook 20 The varactor (or varicap) diode Introduction Many of the circuits for receivers and transmitters presented in this series rely upon the variable capacitor as a means of tuning. Another method of varying capacitance (without any moving parts) is provided by the varactor diode, sometimes called a varicap diode. This is a component which changes its capacitance as the voltage across it is varied. The details Figure 1 shows how a varactor diode might be connected to demonstrate its operation. Its symbol is that of an ordinary diode, with a capacitor symbol next to it. A variable voltage is applied across it in such a way that the diode is reverse-biased. This means that virtually no current passes through it – the positive voltage is applied to the cathode. Varactors are cheaper than variable capacitors, and they are tiny in comparison, very suitable for today’s miniature circuits. If A and B were connected across the tuning coil in a simple receiver (with a series capacitor to block the DC from the battery reaching the coil), the tuning operation would be accomplished by turning the knob on the 10 kilohm potentiometer. Varactors are available with different values, from less than 20 picofarad (pF) for VHF applications to 500 pF for medium-wave radios. They are Figure 1 The capacitance of the varicap diode (between A and B) increases as the voltage is reduced, using the variable resistor 64 A portable radio for medium waves tuned usually by voltages between 2 V and 9 V. For a real application of varactors, you should consult the circuit diagram of the Yearling 20 metre receiver, elsewhere in this book. -

J. W. Miller Company the Coil Forum March 1960
J. W. MILLER COMPANY-LOS ANGELES, CALIFORNIA-VOLUME 1, NUMBER 1 MARCH 1960 A TRANSISTOR FM RECEIVER With this issue, the J. W. Miller Company inaugurates a new publication devoted to the experimenter. Although coils, and their associated com ponents, are our business, we at Miller would like to supply our customers with timely information on circuits, theory to assist with your work or experiments, and data on how to select and use our coils wisely. We believe you will agree that THE COIL FORUM is indeed an appropriate nam~. www.SteamPoweredRadio.Com J. W. M I L L E R C O M P A N Y Recent advances in the state of the semiconductor from the collector back to the base through capacitor art have made it possible to construct a transistor Cl2 and the stage oscillates. Coil L3, along with the FM receiver which performs every bit as well as a tuning capacitor and circuit capacity, determines the vacuum tube version. VHF equipment places very frequency of oscillation, which is always 10. 7 me. strict demands on the transistors. They must be above the incoming signal frequency. Resistors R9 capable of constant amplification over the entire and Rl 1 provide bias and RIO is used for d.c. degener band being received, create a minimum of circuit ation. Transistor QlO and diode Xl are part of the drift, and provide maximum gain with a minimum automatic frequency control system and will be of stages. ' discussed later. All tuned circuits in the "front end" are tracked with a three-gang variable capacitor The excellent performance of the receiver to be (C3, 3-lOmmfd.) described is an outgrowth of the work done by Philco Corporation on their transistor "Safari" tele The i.f. -

Avalanche Diode Detector Unit
A large area avalanche photodiode detector system with USB interface 1. Introduction When measuring low light levels, a vacuum tube photomultiplier tube or some form of solid-state detector which relies on multiplication (e.g. Avalanche diode, Geiger avalanche diode, silicon photomultiplier) is normally used. Vacuum tube photomultipliers have the advantage that a large photosensitive area is available, in contrast to most solid-state devices, which, in general, allow detection over a small area. Avalanche photodiodes make excellent detectors, and here we describe a detector assembly developed around a large area device, 10 mm diameter. This detector unit is designed to detect low light levels and is based around a detector module, commercially available from AP Technology, (www.advancedphotonix.com) part# 197-70-74-661. The detector module is supplied as just that, a small box with flying leads, requiring appropriate low voltage dc power supplies. It does include a thermoelectric cooler and the avalanche diode’s high voltage bias supply. Here we describe how this module was integrated in a photo-detection subsystem, powered from the mains and controlled either from an internal potentiometer or through a computer interface. The completed unit can operate as a stand-alone, manually adjusted unit and powered from a +5V / 2.5A power supply, providing an analogue output in the range 0 to +1V into a 50 Ω (or greater) load over a typical 10 MHz bandwidth. It can also operate as a USB-controlled device, where the detector gain can be remotely set and where output readings and operating conditions can be monitored. -

Capacitor & Capacitance
CAPACITOR & CAPACITANCE - TYPES Capacitor types Listed by di-electric material. A 12 pF 20 kV fixed vacuum capacitor Vacuum : Two metal, usually copper, electrodes are separated by a vacuum. The insulating envelope is usually glass or ceramic. Typically of low capacitance - 10 - 1000 pF and high voltage, up to tens of kilovolts, they are most often used in radio transmitters and other high voltage power devices. Both fixed and variable types are available. Vacuum variable capacitors can have a minimum to maximum capacitance ratio of up to 100, allowing any tuned circuit to cover a full decade of frequency. Vacuum is the most perfect of dielectrics with a zero loss tangent. This allows very high powers to be transmitted without significant loss and consequent heating. Air : Air dielectric capacitors consist of metal plates separated by an air gap. The metal plates, of which there may be many interleaved, are most often made of aluminium or silver-plated brass. Nearly all air dielectric capacitors are variable and are used in radio tuning circuits. Metallized plastic film: Made from high quality polymer film (usually polycarbonate, polystyrene, polypropylene, polyester (Mylar), and for high quality capacitors polysulfone), and metal foil or a layer of metal deposited on surface. They have good quality and stability, and are suitable for timer circuits. Suitable for high frequencies. Mica: Similar to metal film. Often high voltage. Suitable for high frequencies. Expensive. Excellent tolerance. Paper: Used for relatively high voltages. Now obsolete. Glass: Used for high voltages. Expensive. Stable temperature coefficient in a wide range of temperatures. Ceramic: Chips of alternating layers of metal and ceramic. -
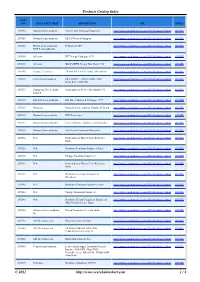
Products Catalog Index
Products Catalog Index PART NO. MANUFACTURER DESCRIPTION URL PRICE 2N5116 National Semiconductor Pro-Electron Transistor Datasheets http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE 2N5116 National Semiconductor JFET SWitches/Choppers http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE 2N5116 Philips Semiconductors / P-Channel JFET http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE NXP Semiconductors 2N5116 Siliconix FET Design Catalogue 1979 http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE 2N5116 Siliconix MOSPOWER Design Data Book 1983 http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE 2N5116 Vishay Telefunken TRANS JFET P-CH 25MA 3TO-206AA http://www.searchdatasheet.com/2N5116-datasheet.html QUOTE 2N5135 Central Semiconductor NPN EPOXY - SWITCHING AND http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE GENERAL PURPOSE 2N5135 Continental Device India Semiconductor Device Data Book 1996 http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE Limited 2N5135 Fairchild Semiconductor Full Line Condensed Catalogue 1977 http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE 2N5135 Motorola Motorola Semiconductor Datasheet Library http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE 2N5135 National Semiconductor NPN Transistors http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE 2N5135 National Semiconductor General Purpose Amplifiers and Switches http://www.searchdatasheet.com/2N5135-datasheet.html QUOTE 2N5135 National Semiconductor Pro-Electron Transistor Datasheets http://www.searchdatasheet.com/2N5135-datasheet.html -
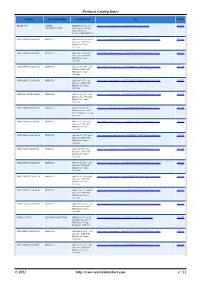
Products Catalog Index
Products Catalog Index PART NO. MANUFACTURER DESCRIPTION URL PRICE DFLR1400-7 DIODES DFLR1400 Series 1 A http://www.searchdatasheet.com/DFLR1400-7-datasheet.html QUOTE INCORPORATED 400 V Surface Mount Glass Passivated Rectifier-POWERDI-123 GRM155R61C474KE01D MURATA 0402 0.47 uF 16 V ±10% http://www.searchdatasheet.com/GRM155R61C474KE01D-datasheet.html QUOTE Tolerance X5R SMT Multilayer Ceramic Capacitor GRM155R60J102KA01D MURATA 0402 1 nF 6.3 V ±10% http://www.searchdatasheet.com/GRM155R60J102KA01D-datasheet.html QUOTE Tolerance X5R SMT Multilayer Ceramic Capacitor GRM155R61C222KA01D MURATA 0402 2.2 nF 16 V ±10% http://www.searchdatasheet.com/GRM155R61C222KA01D-datasheet.html QUOTE Tolerance X5R SMT Multilayer Ceramic Capacitor GRM155R71C102KA01D MURATA 0402 1 nF 16 V ±10% http://www.searchdatasheet.com/GRM155R71C102KA01D-datasheet.html QUOTE Tolerance X7R SMT Multilayer Ceramic Capacitor GRM1885C1H1R5CA01D MURATA 0603 1.5 pF 50 V ±0.25 http://www.searchdatasheet.com/GRM1885C1H1R5CA01D-datasheet.html QUOTE pF Tolerance C0G SMT Multilayer Ceramic Capacitor GRM155R60J334KE01D MURATA 0402 0.33 uF 6.3 V http://www.searchdatasheet.com/GRM155R60J334KE01D-datasheet.html QUOTE ±10% Tolerance X5R SMT Multilayer Ceramic Capacitor GRM155R71H222JA01D MURATA 0402 2.2 nF 50 V ±5% http://www.searchdatasheet.com/GRM155R71H222JA01D-datasheet.html QUOTE Tolerance X7R SMT Multilayer Ceramic Capacitor GRM155R61A103KA01D MURATA 0402 10 nF 10 V ±10% http://www.searchdatasheet.com/GRM155R61A103KA01D-datasheet.html QUOTE Tolerance X5R SMT Multilayer Ceramic -

Circuits for High-Efficiency Avalanche-Diode Oscillators
1060 IEEE TRANSACTIONS ON MICROWAVE THEORY AND TECHNIQUES, VOL. MTT-17, NO. 12, DECEMBER 1969 Circuits for High-Efficiency Avalanche- Diode Oscillators W. J. EVANS Absfract—This paper describes and analyzes the circuits which have II. THE TRAPATT MODE been used successfully for TRAPATT oscillator studies. The results lead In order to understand the operation of high-efficiency to a better understanding of the TRAPATT oscillator and yield a simple model of the oscillator which is useful for circuit design. oscillators, it is necessary to understand what happens when The circuit characteristics of ms experimental TRAPATT oscillator large RF voltage swings exist across an IMPATT diode. are determined from measurements on the circuits and from equivalent Therefore, this section will begin with a review of the large- circuit model calculations. The following conclusions can be drawn from signal behavior of an IMPATT oscillator. the analysis. First, the avalanche diode requires sufficient capacitance near Fig. l(a) is a plot of the field profile and carrier densities the diode to sustain the high-current state required for TRAPATT oper- in an oscillating IMPATT diode at a particular point in ation. Seeondly, at a distance from the diode corresponding to approx. time. The profile shown here is for a 6-GHz n+-p-p+ epi- inmtely one half-wavelength at the TRAPATT frequency the transmission taxial germanium diode [4]. The plots are made for the par- ~me containing the diode should be terminated by a low-pass filter. The ticular point in the RF voltage and current waveforms indi- function of the filter is to pass the TRAPATT frequency and to provide a cated by the squares in Fig. -

Noise in Avalanche Transit-Time Devices
1674 PROCEEDINGS OF THE IEEE, VOL. 59, NO. 12, DECEMBER 1971 for receiving arrays,” ZEEE Trans. Antennas Propagat. (Commun.), [22] C. J. Drane, Jr., and J. F. McIlvenna, “Gain maximization and VO~.AP-14, NOV.1966, pp. 792-794. controlled null placement simultaneously achievedin aerial array [9] A. I. Uzkov, ‘‘An approach to the problem of optimum directive patterns,” Air Force Cambridge Res. Labs., Bedford,Mass., antenna design,” C. R. Acad. Sci. USSR., vol. 35, 1946, p. 35. Rep. AFCRL-69-0257, June 1969. [lo] A. Bloch, R. G. Medhurst, and S. D. Pool, “A new approach to [23] R. F. Hamngton, “Matrixmethods for field problems,” Proc. the design of superdirective aerial arrays,” Proc. Znst. Elec. Eng., ZEEE, vol. 55, Feb. 1967, pp. 136-149. VO~.100, Sept. 1953, pp. 303-314. [24] J. A. Cummins,“Analysis of a circulararray of antennas by [ll] M.Uzsoky and L. Solymar,“Theory of superdirectivelinear matrix methods,” Ph.D. dissertation, Elec. Eng. Dept., Syracuse arrays,” Acta Phys. (Budapt), vol. 6, 1956, pp. 185-204. University, Syracuse,N. Y., Dec. 1968. [12] C. T. Tai, “The optimum directivity of uniformly spaced broad- [25] B. J. Strait and K. Hirasawa, “On radiation and scattering from sidearrays ofdipoles,” ZEEE Trans. Antennas Propgat., vol. arrays of wire antennas,” Proc. Nut. Elec. Con$, vol. 25, 1969. AP-12, July 1964, pp. 447-454. [26] A.T. Adams and B. J. Strait, “Modernanalysis methods for [13] D. K. Cheng and F. I. Tseng, “Gain optimization for arbitrary EMC,” ZEEEIEMC Symp. Rec., July 1970, pp. 383-393. antenna arrays,’’ ZEEE Trans.