Chinese Medicine Biomed Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
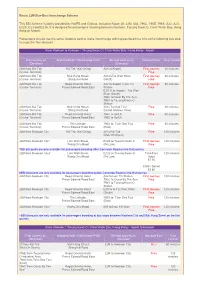
Route 22M Bus-Bus Interchange Scheme
Route 22M Bus-Bus Interchange Scheme This BBI Scheme is jointly provided by NWFB and Citybus, including Route 20, 22M, 608, 796C, 796E, 796X, A22, A23, E22S, E23 and E23A. It is designed for passengers traveling between Kowloon, Tseung Kwan O, Clear Water Bay, Hong Kong or Airport. Passengers should use the same Octopus card to make interchange within prescribed time limit at the following bus stop to enjoy the fare discount. From Kowloon to Kowloon / Tseung Kwan O / Clear Water Bay / Hong Kong / Airport First Journey on Alighting Point / Interchange Point Second Journey on Discount Fare Time Limited (Direction) (Direction) 22M from Kai Tak Kai Tak (Kai Ching) A23 to Airport First Journey 60 minutes (Cruise Terminal) Free 22M from Kai Tak Muk Hung Street, A23 toTsz Wan Shan First Journey 60 minutes (Cruise Terminal) Shing Kai Road (North) Free 22M from Kai Tak Regal Oriental Hotel, A22 to Airport / Lam Tin First Journey 60 minutes (Cruise Terminal) Prince Edward Road East Station Free E23/ A to Airport / Tsz Wan Shan (South) 796C toOscar By The Sea 796X to Tseung Kwan O Station 22M from Kai Tak Muk Hung Street, 20 to Tai Kok Tsui Free 60 minutes (Cruise Terminal) Shing Kai Road (Island Harbour View) 22M from Kai Tak Regal Oriental Hotel, 796C to So Uk Free 60 minutes (Cruise Terminal) Prince Edward Road East 796E to So Uk 22M from Kai Tak The Latitude, 796X to Tsim Sha Tsui Free 60 minutes (Cruise Terminal) Prince Edward Road East (East) 22M from Kowloon City Kai Tak (Kai Ching) 20 to Kai Tak Free 120 minutes (Muk On Street) 22M from -

GEO REPORT No. 282
EXPERT REPORT ON THE GEOLOGY OF THE PROPOSED GEOPARK IN HONG KONG GEO REPORT No. 282 R.J. Sewell & D.L.K. Tang GEOTECHNICAL ENGINEERING OFFICE CIVIL ENGINEERING AND DEVELOPMENT DEPARTMENT THE GOVERNMENT OF THE HONG KONG SPECIAL ADMINISTRATIVE REGION EXPERT REPORT ON THE GEOLOGY OF THE PROPOSED GEOPARK IN HONG KONG GEO REPORT No. 282 R.J. Sewell & D.L.K. Tang This report was originally produced in June 2009 as GEO Geological Report No. GR 2/2009 2 © The Government of the Hong Kong Special Administrative Region First published, July 2013 Prepared by: Geotechnical Engineering Office, Civil Engineering and Development Department, Civil Engineering and Development Building, 101 Princess Margaret Road, Homantin, Kowloon, Hong Kong. - 3 - PREFACE In keeping with our policy of releasing information which may be of general interest to the geotechnical profession and the public, we make available selected internal reports in a series of publications termed the GEO Report series. The GEO Reports can be downloaded from the website of the Civil Engineering and Development Department (http://www.cedd.gov.hk) on the Internet. Printed copies are also available for some GEO Reports. For printed copies, a charge is made to cover the cost of printing. The Geotechnical Engineering Office also produces documents specifically for publication in print. These include guidance documents and results of comprehensive reviews. They can also be downloaded from the above website. The publications and the printed GEO Reports may be obtained from the Government’s Information Services Department. Information on how to purchase these documents is given on the second last page of this report. -

The Hong Kong University of Science & Technology
EXCHANGE FACT SHEET (2015-2016) The Hong Kong University of Science & Technology Address HKUST MBA Program Office Room 2011, Lifts 1/2 Lee Shau Kee Business Building The Hong Kong University of Science and Technology Clear Water Bay Kowloon HONG KONG Contact Miss Michelle To Assistant Manager, MBA Student Development Miss Ivy Leung & Miss Mandy Lee Exchange Assistant, MBA Student Development Telephone (852) 2358 5980 / 2358 7545 Fax (852) 2705 9596 Email [email protected] MBA Program Website www.mba.ust.hk Exchange Program Web Links http://www.mbanet.ust.hk (for exchange/visiting students) Exchange-in Preparation Download forms for exchange-related documents Course Schedule of current semester (Update version available in July) Full access will be granted upon your receipt of HKUST email account HKUST HKUST is the third publicly-funded university in Hong Kong. It was incorporated in April 1988 and opened in October 1991 as a world-class technological university dedicated to the advancement of learning and scholarship, with special emphasis on research and postgraduate education. Current student enrolment at the undergraduate, master’s and doctoral levels stands at 13,000. The Campus The University’s campus occupies a 60-hectare of sweeping beauty on the Clear Water Bay Peninsula of Sai Kung. The new international airport at Chek Lap Kok is about 45 kilometers from HKUST. Visitors can come to the campus by bus, Airport Express train or taxi. It is about 60 minutes to 90 minutes from the airport. Visitors to HKUST are often overwhelmed by the beauty of the architecture in its natural surroundings. -

Shaw Brothers
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION If you are in any doubt as to any aspect of this document or as to the action to be taken, you should consult a licensed securities dealer or registered institution in securities, a bank manager, solicitor, professional accountant or other professional adviser. If you have sold or otherwise transferred all your shares in Shaw Brothers (Hong Kong) Limited, you should at once hand this document and the accompanying forms of proxy to the purchaser(s) or transferee(s) or to the licensed securities dealer or registered institution in securities or other agent through whom the sale or transfer was effected for transmission to the purchaser(s) or transferee(s). Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this document, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this document. Shaw Holdings Inc. Shaw Brothers (Hong Kong) Limited (incorporated in the Republic of Nauru) (incorporated in Hong Kong with limited liability) (Stock Code: 00080) PROPOSAL TO PRIVATISE SHAW BROTHERS (HONG KONG) LIMITED BY WAY OF A SCHEME OF ARRANGEMENT UNDER SECTION 166 OF THE COMPANIES ORDINANCE AND PROPOSED WITHDRAWAL OF LISTING OF SHAW BROTHERS (HONG KONG) LIMITED Financial adviser to Shaw Holdings Inc. Independent financial adviser to the Independent Board Committee of Shaw Brothers (Hong Kong) Limited A letter from the Board is set out on pages 8 to 14 of this document. -

Sunset Peak Is Famous for Its Stunning Sunset Views and Seas of Silvergrass, Especially in Autumn
A SENSE OF PLACE Being outdoors has important effects on our smells of the forest, or of drying fish and mental and physical wellbeing, especially shrimp paste in a traditionalvillage; visit when we are active, such as when we are shorelines where you can touch rocks that bear hiking. Though Hong Kong is thought of as a the scars of a volcanic past. concrete jungle, its density means that the wild outdoors is closer to downtown streets than it Engaging your senses like this is a powerful is in other parts of the world so those healthy way to create shared memories withfriends escapes are easily attained. and family. It also shows how Hong Kong’s countryside is not a secondaryattraction but Once there, you can open your senses wide. rather is key to the city’s appeal. Gaze back at the city skyline seenfrom the mountains; listen to waves crashing on remote Now, let’s indulge our sense of touch as beaches; savour the taste oflocal dishes we enjoy some of Hong Kong’s outdoor that connect you with Hong Kong’s cultural playgrounds. heritage; take a deep breathand absorb the Discover Hong Kong © Copyright Hong Kong Tourism Board 2020 1 2 GREAT OUTDOORS HONG KONG HIKING & CYCLING GUIDEBOOK TIPS & GEAR Check out these hiking tips and our recommended gear checklist to help you have a safe and enjoyable hike. Open your senses FOOD & DRINK and go explore! Never eat or drink while moving. Never drink untreated water from hill streams or eat any wild plants or mushrooms. Don’t consume icy drinks immediately after a long hike, when your PACKING body temperature is still high. -

Office Address of the Labour Relations Division
If you wish to make enquiries or complaints or lodge claims on matters related to the Employment Ordinance, the Minimum Wage Ordinance or contracts of employment with the Labour Department, please approach, according to your place of work, the nearby branch office of the Labour Relations Division for assistance. Office address Areas covered Labour Relations Division (Hong Kong East) (Eastern side of Arsenal Street), HK Arts Centre, Wan Chai, Causeway Bay, 12/F, 14 Taikoo Wan Road, Taikoo Shing, Happy Valley, Tin Hau, Fortress Hill, North Point, Taikoo Place, Quarry Bay, Hong Kong. Shau Ki Wan, Chai Wan, Tai Tam, Stanley, Repulse Bay, Chung Hum Kok, South Bay, Deep Water Bay (east), Shek O and Po Toi Island. Labour Relations Division (Hong Kong West) (Western side of Arsenal Street including Police Headquarters), HK Academy 3/F, Western Magistracy Building, of Performing Arts, Fenwick Pier, Admiralty, Central District, Sheung Wan, 2A Pok Fu Lam Road, The Peak, Sai Ying Pun, Kennedy Town, Cyberport, Residence Bel-air, Hong Kong. Aberdeen, Wong Chuk Hang, Deep Water Bay (west), Peng Chau, Cheung Chau, Lamma Island, Shek Kwu Chau, Hei Ling Chau, Siu A Chau, Tai A Chau, Tung Lung Chau, Discovery Bay and Mui Wo of Lantau Island. Labour Relations Division (Kowloon East) To Kwa Wan, Ma Tau Wai, Hung Hom, Ho Man Tin, Kowloon City, UGF, Trade and Industry Tower, Kowloon Tong (eastern side of Waterloo Road), Wang Tau Hom, San Po 3 Concorde Road, Kowloon. Kong, Wong Tai Sin, Tsz Wan Shan, Diamond Hill, Choi Hung Estate, Ngau Chi Wan and Kowloon Bay (including Telford Gardens and Richland Gardens). -

Grading of Beach Water Quality Released
Grading of beach water quality released The Environmental Protection Department (EPD) today (August 27) released the latest grading of water quality for 39 gazetted beaches (see Note 1) and one non-gazetted beach (i.e. Discovery Bay, see Note 2). Twenty-two beaches were rated as Good (Grade 1), 15 as Fair (Grade 2) and three as Poor (Grade 3). Grade 1 beaches are: Cafeteria New Beach Repulse Bay Beach* Cheung Chau Tung Wan Beach* Shek O Beach* Chung Hom Kok Beach Silverstrand Beach* Clear Water Bay First Beach South Bay Beach Clear Water Bay Second Beach* St Stephen's Beach Discovery Bay Stanley Main Beach* Golden Beach* Tai Po Lung Mei Beach* Hap Mun Bay Beach* Tong Fuk Beach Hung Shing Yeh Beach* Trio Beach Kiu Tsui Beach Turtle Cove Beach Lo So Shing Beach Upper Cheung Sha Beach Grade 2 beaches are: Anglers' Beach Kwun Yam Beach Approach Beach Lido Beach* Cafeteria Old Beach Lower Cheung Sha Beach Casam Beach* Ma Wan Tung Wan Beach* Castle Peak Beach Middle Bay Beach Deep Water Bay Beach* Pui O Beach* Hoi Mei Wan Beach Ting Kau Beach Kadoorie Beach Grade 3 beaches are: Big Wave Bay Beach* Silver Mine Bay Beach* Butterfly Beach* Compared with the grading released last week, Cheung Chau Tung Wan Beach, Clear Water Bay Second Beach, Kiu Tsui Beach, Silverstrand Beach and Tai Po Lung Mei Beach have been upgraded from Grade 2 to Grade 1; Casam Beach and Ting Kau Beach from Grade 3 to Grade 2. Middle Bay Beach has been changed from Grade 1 to Grade 2. -

Recreation, Sport and the Arts
367 Chapter 19 Recreation, Sport and the Arts Hong Kong is well known for its hard- working people, but it is not an all-work- no-play city. People spend time in a wide variety of recreational, sport and cultural activities, ranging from ‘tai chi’ to yoga, football to rugby, and international arts festivals to home-grown performances. Hong Kong offers many opportunities for people to unwind. Recreation, sport and the arts provide an opportunity for people in Hong Kong to improve their quality of life. The Government helps to nurture an environment in which creative freedom, a pluralist approach to the development of the arts, sporting excellence and recreation can thrive. Government policies on matters concerning sport, recreation, culture and heritage are coordinated by the Home Affairs Bureau. Organisations that help to draw up these policies include the Hong Kong Sports Commission, the Hong Kong Sports Institute, the former Culture and Heritage Commission, the Hong Kong Arts Development Council and the Antiquities Advisory Board. The Hong Kong Sports Institute Limited was set up as a delivery agent to help develop sports in Hong Kong with special emphasis on training athletes for high- performance sports. In January 2005, the Government established the Sports Commission to advise on all matters related to sports development. The commission oversees the Elite Sports Committee, the Major Sports Events Committee and the Community Sports Committee which give advice on different aspects of sporting activities. The new advisory structure is a milestone for sports development in Hong Kong. The Leisure and Cultural Services Department (LCSD), an executive arm of the Home Affairs Bureau, provides leisure and cultural services to the community, preserves its cultural heritage, beautifies its physical environment, and fosters synergy among sports, cultural and community organisations. -

Traffic Advice KMB Recreation Service Route No. 91R Members of Public Are Advised That KMB Route No. 91R Will Be Operated from 2
Traffic Advice KMB Recreation Service Route No. 91R Members of public are advised that KMB Route No. 91R will be operated from 25 June 2020 to 30 August 2020. The service details of the route are as follows: Route: KMB Route No. 91R (Clear Water Bay to Choi Ming) Routeing: Tai O Mun Road, Clear Water Bay Road, Hang Hau Road, Po Ning Road, Sheung Ning Road, Chung Wa Road, Pui Shing Road, Ngan O Road, Chiu Shun Road, Po Yap Road, Tong Chun Street, Tong Ming Street, Po Shun Road, Chui Ling Road, King Ling Road, Tiu Keng Leng Public Transport Interchange, King Ling Road and Choi Ming Street. Headways: 30 mins Service From 5:00 p.m. to 7:30 p.m. on Sundays and Public Holidays only Hours and between 25 June 2020 and 30 August 2020 Period: Fare: $10.50 per single journey Bus Stops: 1. Clear Water Bay Second Beach Public Transport Interchange 2. Tai Au Mun Road near Clear Water Bay First Beach 3. Tai Au Mun Road opposite to Tai Wan Tau Road 4. Clear Water Bay Road opposite to Sheung Sze Wan Road 5. Clear Water Bay Road opposite to Leung Fai Tin Lower Road 6. Clear Water Bay Road opposite to Ha Yeung Village 7. Clear Water Bay Road opposite to Sheung Yeung Village 8. Clear Water Bay Road opposite to Pan Long Wan Road 9. Clear Water Bay Road outside High Junk Peak 10. Clear Water Bay Road near Mang Kung Uk Village 11. Clear Water Bay Road near Mang Kung Uk Road 12. -

Tung Chung to Tai O
A SENSE OF PLACE Being outdoors has important effects on our smells of the forest, or of drying fish and mental and physical wellbeing, especially shrimp paste in a traditionalvillage; visit when we are active, such as when we are shorelines where you can touch rocks that bear hiking. Though Hong Kong is thought of as a the scars of a volcanic past. concrete jungle, its density means that the wild outdoors is closer to downtown streets than it Engaging your senses like this is a powerful is in other parts of the world so those healthy way to create shared memories withfriends escapes are easily attained. and family. It also shows how Hong Kong’s countryside is not a secondaryattraction but Once there, you can open your senses wide. rather is key to the city’s appeal. Gaze back at the city skyline seenfrom the mountains; listen to waves crashing on remote Now, let’s indulge our sense of smell as we beaches; savour the taste oflocal dishes enjoy the recommended hiking trail. that connect you with Hong Kong’s cultural heritage; take a deep breathand absorb the Discover Hong Kong © Copyright Hong Kong Tourism Board 2020 1 2 GREAT OUTDOORS HONG KONG HIKING & CYCLING GUIDEBOOK TIPS & GEAR Check out these hiking tips and our recommended gear checklist to help you have a safe and enjoyable hike. Open your senses FOOD & DRINK and go explore! Never eat or drink while moving. Never drink untreated water from hill streams or eat any wild plants or mushrooms. Don’t consume icy drinks immediately after a long hike, when your PACKING body temperature is still high. -

M / SP / 14 / 142 CLEAR WATER BAY ROAD J⁄� Q¼j M†�⁄W˘¥ TRIO ISLAND 333 MIU TSAI TUN CLEAR WATER BAY (TAI LAK LEI) SEE PLAN REF
182 100 ¥F 78 Pak Sha Tsui 100 _¥ 1 8 205 ¥ 1 Pak A ¥ 10 TAI NGAM PING PAI j¤¥ G¤ 100 HAU WHISKEY 13 Pak Lap Tai Ngam Tsui s·† BEACH YI LENG KAM LO WAN 3 PAK LAP TSAI 1 1 8.2.1 Lung Ngan «¯P¥ 12 210 D³W w¬ Yuen Tau SAN SIN TSENG SHAN SHE WAN SHAN6 5 j¤ CHAU TSAI 100 i±½ 124 66 1 217 TAI LENG 100 CHEUNG KUNG 2 PAK LAP WAN SHAN 8.2 ¥¤ Pak Ma Tsui 4 200 2 100 j¤¥ KAU CHUNG 1 WAN TAI NGAM TENG SHEK TSAI 8.2.3 ] WAN Mau Tin 86 1 F¤ 11 1 2 CAMPERS' BEACH ¥¤ 100 Sha Kiu Tau Pak Ma Tsui Pai Fª KAU TUNG Tung A 100 WAN D³W 4 She Wan Kok 119 KAU SAI ¶d WAN 23 4 WONG NAI CHAU ] Kau Sai 8.1.3 156 5 7 100 n«· ¥Œ NAM FUNG 5 j¤ PAK FU SHAN ¶f CHAU 5 êÂÍ Tai Pai Tong Hau Pai Sor See Kau MA TAU WAN PAK SHUI WUN KAU SHUI LIU ROCKY HARBOUR ( LEUNG SHUEN WAN HOI ) PORT SHELTER 4 ú¥Y 19 ( NGAU MEI HOI ) KONG TAU PAI 4 NAI MANG PO 96 »›·‹ ˝ ł¥Y SOR SEE MUN The Hong Kong University 200 3 Y– KEI TAU KOK TENG of Science and Technology BAY ISLET Q¦ÁÄ CHEUNG WAN 73 BUN BEI CHAU TIU CHUNG TAI SHAN (SEE CHAU) 22 SEE CHAU MUN 2 20 216 4 4 100 4 2 Q¦Á 3 º¥Y 24 JIN ISLAND §r‹ TOWN ISLAND ø¥ Addiction (FO TAU FAN CHAU) 4 (TIU CHUNG CHAU) KEI SHAN Treatment Centre 42 “⁄ 179 Shaw Studio ø¥s 鮨 100 A KUNG WAN KEI SHAN AU Wai Kap Pai TAI WONG WAN û½ 3 4 5 ” Kai Yi Wan 3 Bella Vista 3 3 100 ª¨¤ WANG CHAU 8.1 Chek Kok Ngam 95 TAI WONG “` w WAN ¹©W Kam Chung Ngam Wang Chau Kok 189 2 SAM CHAU MUN fi Wong Wan Pai The Villa Horizon 117 4 « ê¶^ ß⁄ Q¦Á PIN CHAU 8.3 |Âû¬s¸q® YUEN KONG 4 4 Tiu Chung2 Pai 8.1.4 20 SHELTER ISLAND UNG KONG GROUP CHAU 2 (NGAU MEI CHAU) SPECIAL AREA -

Pre 1:40000 P1 Locations of Key Water Sensitive Receivers
'l⁄ ^·” Pat Tsz Wo Penfold Park ‡_ Wong Chuk Wan TAI MONG TSAI Village¥d A KUNG KOK Nam A Wo Liu Hang LUK CHAU AU Long Keng 414 j⁄ Wo Liu FO TAN Tai Po 445 ‡_ PYRAMID HILL Tsai She Tau LUK CHAU SHAN ( TAI KAM CHUNG ) ¶¸ 536 314 Wong Chuk Yeung X¼ Cemetery ” Shan Liu LEGEND: õ¤´ ł¶B„¤N‡æ⁄` Pictorial Garden Tso Wo Hang t Fo Tan Village Olympic Equestrian Venue Ser Res ( Sha Tin ) ¥b ¤bs⁄¥ Lung Mei Tai Wan Shek Lung Tsai A»· Ngau Liu San Tin Hang Tai Mong Garden Vista Kak Hang Tun Tsai A` 281 J` MA ON SHAN COUNTRY PARK Lookout Sui Wo |fi k¤C Fu Tei SITE BOUNDARY t Court ”· NUI PO SHAN399 –l Keng Ser Res A A` Ravana Garden Mui Tsz Lam Pang Ha Hau Greenwood Terrace SHEK MUN A` Long Mei Lookout U⁄ ù© Lookout San Uk Ha Wo Che Ngong Ping “T Fu Yung Pit Muk Min Wo Tong Shan Tai Chau ƱY ¥| k¤C Kong w Pai Tau Hang NUI PO AU Sha Ha NEEDLE HILL W⁄ ¥bˆJ Nam Shan FLUSHING WATER INTAKE ͤR Sheung Wo Che Wo Che Shek Lung Tsai Sha Kok Mei Outward Bound Yau Oi Tsuen Estate F¨Ð¥Ä New Village School 532 û¤ Lap Sap SHING MUN Ngau Au TW© j⁄ Kap Pin Long City One Shatin New Village Chau RESERVOIR n« Mau Ping 372 Tai Shui Tseng WSD1 - KOWLOON SOUTH wý TAI SHEK KWU Nam Shan Lo Uk D¹· Lek Yuen Kap Pin Long To Fung Shan Estate C Ʊ aª r´Ð ¥j T M©y t Pai Tau Sw P Shek Kwu Lung Ser Res YUEN Yue Tin «ø TW© Mau Ping 314 Wo Yi Hop Court j¤Å Mau Ping Tan Cheung CHAU KOK Castello Tai Lam Liu 300 ⁄ 70 San Uk A» Ð¥ Wong Uk ¶d ¥ Kwun Tsoi Pai Tin Liu s•«« p¤w Wong Nai Tau SHEK NGA SHAN Yau Ma Po New Town Siu Lek Tai Ping ‹Q Pristine Villa Plaza s¼½ j¤ 540 j¤| r´A Yuen