Prevalence of Bacterial Wilt of Ginger (Z
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Inter Aide Ethiopia
Inter Aide Ethiopia TERMINAL EVALUATION REPORT ACP-EU Water Facility Project Sustainable Access to Safe Water and Basic Sanitation Services Through Improved Capacities of the Community Based and Local Institutional Actors SNNP Region Girma Mengistu [T.G.M. Consultancy] Tel. 0911 14 34 22, 011 439 26 56 Email: [email protected] P.O. Box: 93, Kaliti, Addis Ababa, Ethiopia January, 2013 Addis Ababa Inter Aide Terminal Evaluation of WASH Program in SNNP Region Final Report TABLE OF CONTENTS ACRONYMS .................................................................................................................. III LIST OF TABLES, FIGURES AND PICTURES .................................................................... IV EXECUTIVE SUMMARY ................................................................................................................. V 1 INTRODUCTION .................................................................................................................... 1 1.1 COUNTRY AND SECTOR CONTEXTUAL BACKGROUND ......................................... 1 1.2 TERMINAL EVALUATION OBJECTIVES AND METHODOLOGY .................................. 3 2 THE PROGRAM AND ITS RELEVANCE .............................................................................. 7 2.1 THE PROGRAM AREA ........................................................................................ 7 2.2 THE PROPOSED PROGRAM ................................................................................ 9 2.3 RELEVANCE OF THE PROGRAM ....................................................................... -

Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING GEOGRAPHICALLY WEIGHTED REGRESSION Berhanu Berga Dadi i MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING THE GEOGRAPHICALLY WEIGHTED REGRESSION MODEL, 2015-2016 Dissertation supervised by Dr.Jorge Mateu Mahiques,PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain Ana Cristina Costa, PhD Professor, Nova Information Management School University of Nova Lisbon, Portugal Pablo Juan Verdoy, PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain March 2020 ii DECLARATION OF ORIGINALITY I declare that the work described in this document is my own and not from someone else. All the assistance I have received from other people is duly acknowledged, and all the sources (published or not published) referenced. This work has not been previously evaluated or submitted to the University of Jaume I Castellon, Spain, or elsewhere. Castellon, 30th Feburaury 2020 Berhanu Berga Dadi iii Acknowledgments Before and above anything, I want to thank our Lord Jesus Christ, Son of GOD, for his blessing and protection to all of us to live. I want to thank also all consortium of Erasmus Mundus Master's program in Geospatial Technologies for their financial and material support during all period of my study. Grateful acknowledgment expressed to Supervisors: Prof.Dr.Jorge Mateu Mahiques, Universitat Jaume I(UJI), Prof.Dr.Ana Cristina Costa, Universidade NOVA de Lisboa, and Prof.Dr.Pablo Juan Verdoy, Universitat Jaume I(UJI) for their immense support, outstanding guidance, encouragement and helpful comments throughout my thesis work. Finally, but not least, I would like to thank my lovely wife, Workababa Bekele, and beloved daughter Loise Berhanu and son Nethan Berhanu for their patience, inspiration, and understanding during the entire period of my study. -

Tulu Kapi Nyota Min Ltd '09 Technical
- Key Features - Exploration - Introduction - Mineralisation - Project Description, General Infrastructure and - Distribution of Mineralistation Accessibility - Deposit Type - Topography, Climate and Vegetation - Sampling Method and Approach - Legal Aspects and Tenure - Sample Preparation, Analysis and Security - Environmental Requirements - Mineralogical Studies and Mineral Processing - Country History - Data Verification and QA/QC - Country Profile and Economy - Adjacent Properties and Competitor Companies - Mining Sector of the Economy - Modelling and Mineral Resource Estimation IN THIS DOCUMENT - Historical Exploration and Operations in the Tulu - Conclusions Investors Report on the Kapi Area - References Tulu Kapi Gold Project, Ethiopia - Regional Geological Setting th - Local Geology as at 30 September 2009 KEY FEATURES Compliance: Venmyn utilises a comprehensive checklist incorporating all internationally required compliance requirements, in particular the Canadian National Instrument 43-101 and SAMREC/SAMVAL Codes for public reporting of mineral assets. The information quoted in this Prospectivity Review has been scrutinised in terms of this checklist and prepared for investors according to the principles of open and transparent disclosure embodied in the underlying codes for mineral resources reporting. Qualified Persons: Mr.Andy Clay, M.Sc. (Geol), M.Sc. (Min. Eng.), Dip.Bus.M., Pr.Sci.Nat., MSAIMM, FAusIMM, FGSSA,AAPG, M.Inst.D. Mr. Neil Mc Kenna, M.Sc. (Geol), Pr.Sci.Nat., MSAIMM, MGSSA, MIASSA, M.Inst.D. Mr. Richard Tayelor, B.Sc. Hons (Geol). MGSSA. Effective Date: 30th September 2009. Prepared For: Nyota Minerals Limeted (Nyota), previously Dwyka Resources Limited (Dwyka). Purpose: Review of the prospectivity and technical merits of the Tulu Kapi Gold Project in Ethiopia. Sources of Information: Public domain information as listed in the reference list, Nyota, Dwyka and Minerva Resources PLC internal reports and, presentations and Hellman & Schofield (Pty) Ltd. -

Determinants of Dairy Product Market Participation of the Rural Households
ness & Fi si na u n c B Gemeda et al, J Bus Fin Aff 2018, 7:4 i f a o l l A a Journal of f DOI: 10.4172/2167-0234.1000362 f n a r i r u s o J ISSN: 2167-0234 Business & Financial Affairs Research Article Open Access Determinants of Dairy Product Market Participation of the Rural Households’ The Case of Adaberga District in West Shewa Zone of Oromia National Regional State, Ethiopia Dirriba Idahe Gemeda1, Fikiru Temesgen Geleta2 and Solomon Amsalu Gesese3 1Department of Agricultural Economics, College of Agriculture and Veterinary Sciences, Ambo University, Ethiopia 2Department of Agribusiness and Value Chain Management, College of Agriculture and Veterinary Sciences, Ambo University, Ethiopia Abstract Ethiopia is believed to have the largest Livestock population in Africa. Dairy has been identified as a priority area for the Ethiopian government, which aims to increase Ethiopian milk production at an average annual growth rate of 15.5% during the GTP II period (2015-2020), from 5,304 million litters to 9,418 million litters. This study was carried out to assess determinants of dairy product market participation of the rural households in the case of Adaberga district in West Shewa zone of Oromia national regional state, Ethiopia. The study took a random sample of 120 dairy producer households by using multi-stage sampling procedure and employing a probability proportional to sample size sampling technique. For the individual producer, the decision to participate or not to participate in dairy production was formulated as binary choice probit model to identify factors that determine dairy product market participation. -

Aalborg Universitet Restructuring State and Society Ethnic
Aalborg Universitet Restructuring State and Society Ethnic Federalism in Ethiopia Balcha, Berhanu Publication date: 2007 Document Version Publisher's PDF, also known as Version of record Link to publication from Aalborg University Citation for published version (APA): Balcha, B. (2007). Restructuring State and Society: Ethnic Federalism in Ethiopia. SPIRIT. Spirit PhD Series No. 8 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. ? Users may download and print one copy of any publication from the public portal for the purpose of private study or research. ? You may not further distribute the material or use it for any profit-making activity or commercial gain ? You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us at [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from vbn.aau.dk on: November 29, 2020 SPIRIT Doctoral Programme Aalborg University Kroghstraede 3-3.237 DK-9220 Aalborg East Phone: +45 9940 9810 Mail: [email protected] Restructuring State and Society: Ethnic Federalism in Ethiopia Berhanu Gutema Balcha SPIRIT PhD Series Thesis no. 8 ISSN: 1903-7783 © 2007 Berhanu Gutema Balcha Restructuring State and Society: Ethnic Federalism in Ethiopia SPIRIT – Doctoral Programme Aalborg University Denmark SPIRIT PhD Series Thesis no. -

Impact of Deforestation on Biodiversity, Soil Carbon Stocks, Soil Quality, Runoff and Sediment Yield at Southwest Ethiopia’S Forest Frontier
Impact of deforestation on biodiversity, soil carbon stocks, soil quality, runoff and sediment yield at southwest Ethiopia’s forest frontier Henok Kassa Tegegne Proefschrift voorgedragen tot het behalen van de graad van Doctor in de Wetenschappen Geografie Faculteit Wetenschappen Henok Kassa Tegegne Impact of deforestation on biodiversity, soil carbon stocks, soil quality, runoff and sediment yield at southwest Ethiopia’s forest frontier Proefschrift voorgelegd tot het behalen van de graad van Doctor in de Wetenschappen: Geografie 2016-2017 Copyright: Henok Kassa 2017 Published by: Department of Geography - Ghent University Krijgslaan 281 (S8), 9000 Gent (Belgium) (c) All rights reserved. ix x Promoter: Prof. Dr. Jan Nyssen, Department of Geography, Faculty of Sciences, Ghent University, Belgium Co-promoter: Prof. Dr. Jean Poesen, Department of Earth and Environmental Sciences, Section of Geography and Tourism, KU Leuven, Belgium Members of the Jury: Prof. Dr. Nico Vandeweghe, Department of Geography, Faculty of Sciences, Ghent University, Belgium (Chair) Dr. Denyse Snelder, Senior Advisor Natural Resources Management, VU Amsterdam, The Netherlands Prof. Dr. Stefaan Dondeyne, Department of Earth and Environmental Sciences, Section of Soil and Water Management, KU Leuven, Belgium Prof. Dr. Ann Verdoodt, Department of Soil Management, Faculty of Biosciences Engineering, Ghent University, Belgium Dr. Amaury Frankl, Department of Geography, Faculty of Sciences, Ghent University, Belgium (secretary) Dr. Miro Jacob, Department of Geography, Faculty of Sciences, Ghent University, Belgium Dean: Prof. Dr. Herwig Dejonghe Rector: Prof. Dr. Anne De Paepe xi xii Acknowledgements First and foremost, I thank the Almighty God for granting me the capability and patience to accomplish the study. Firstly, I would like to express my sincere gratitude to my promoters Prof. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

190327 Oromia Region Agric S
ETHIOPIA: AGRICULTURE SECTOR HRP OROMIA REGION MONTHLY DASHBOARD - March 2019 The devastating impact on agriculture following consecutive years of drought in Ethiopia is undisputed. While forecasts for 2019 indicate a probability of normal to above normal rain in most parts of Ethiopia, in east, south and southeastern regions, the upcoming rainy season (March to June) is forecast- KEY FIGURES ed to be average or below average. In areas where normal to above normal rains are expected, recovery will not be spontaneous, as previous OVERVIEW HOUSEHOLDS REACHED drought-affected households are likely to require sustained humanitarian assistance as a result of exhausted coping mechanisms. HOUSEHOLDS IN NEED Humanitarian assistance for IDPs and IDP returnees is largely dependent on IDPs’ access to land and the livelihood assets they have been able to 1.15 million maintain during displacement. Emergency feed and animal health interventions are needed to reduce the burden on the resources of the host 0.0m 0% communities and prevent the spread of diseases, especially for animals displaced across regional borders. Where appropriate, land will be availed and crop seeds, farming tools, and training will be provided to support IDP and returning households to improve their food security and reduce the burden HOUSEHOLDS TARGETED on host Communities. 658,428 IDP HOUSEHOLDS TARGETED N_Shewa 0m 0% Horo 64,195 Guduru Chinaksen Guto W_Wellega Gida Meta Sasiga Finfine Doba Gursum AGRICULTURE LIVESTOCK E_Wellega Mieso Crop Seeds &Tools W_Shewa Special Girawa -

Wild Animal Status and Their Threats in Echefa
sity & En er da v n i g d e Journal of Biodiversity & Endangered o i r e Wale M et al., J Biodivers Endanger Species 2018, B d f S o 6:3 p l e a c ISSN:n 2332-2543 r i Species DOI: 10.4172/2332-2543.1000222 e u s o J Research Open Access Wild Animal Status and their Threats in Echefa Forest and Wetland (Proposed In-situ Conservation Site), Southern Nations Nationalities and People’Regionals States, Ethiopia Mengistu Wale*, Abeje Kassie, Weldemariam Tesfahunegny and Gebregziabher Hailay Ethiopian Biodiversity Institute Addis Ababa, Ethiopia *Corresponding author: Mengistu Molaliegnwale, Ethiopian Biodiversity Institute Addis Ababa, Ethiopia, E-mail: [email protected] Received date: July 23, 2018; Accepted date: September 30, 2018; Published date: October 15, 2018 Copyright: © 2018 Molaliegnwale M. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Assessment of wild animal diversity and their threats was carried out from April 2017 to December 2018 in proposed in situ conservation area ‘Echefa forest and wetlands’, which is adjustment to Kaffa Biosphere Reserve, Southwest Ethiopia. Data were collected using semi-structured questionnaire through interview of selected 112 local communities, focus group discussion and direct observation. The data analyzed using descriptive statistics. A total of 146 wild animals, 24 mammals, 70 bird species, 22 herpetofauna and 30 insect species were identified. However, the wild animal resources declined 99% in the last twenty years due to illegal hunting 68.8% followed by habitat loss 22.3%. -
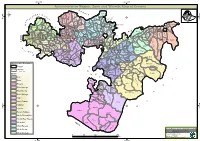
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

The Impacts of Microcredit: Evidence from Ethiopia†
American Economic Journal: Applied Economics 2015, 7(1): 54–89 http://dx.doi.org/10.1257/app.20130475 The Impacts of Microcredit: Evidence from Ethiopia† By Alessandro Tarozzi, Jaikishan Desai, and Kristin Johnson* We use data from a randomized controlled trial conducted in 2003–2006 in rural Amhara and Oromiya Ethiopia to study the impacts of increasing access to microfinance( on a) number of socioeconomic outcomes, including income from agriculture, animal husbandry, nonfarm self-employment, labor supply, schooling and indicators of women’s empowerment. We document that despite sub- stantial increases in borrowing in areas assigned to treatment the null of no impact cannot be rejected for a large majority of outcomes. JEL G21, I20, J13, J16, O13, O16, O18 ( ) eginning in the 1970s, with the birth of the Grameen Bank in Bangladesh, Bmicrocredit has played a prominent role among development initiatives. Many proponents claim that microfinance has had enormously positive effects among borrowers. However, the rigorous evaluation of such claims of success has been complicated by the endogeneity of program placement and client selection, both common obstacles in program evaluations. Microfinance institutions MFIs typi- ( ) cally choose to locate in areas predicted to be profitable, and or where large impacts / are expected. In addition, individuals who seek out loans in areas served by MFIs and that are willing and able to form joint-liability borrowing groups a model often ( preferred by MFIs are likely different from others who do not along a number of ) observable and unobservable factors. Until recently, the results of most evaluations could not be interpreted as conclusively causal because of the lack of an appropriate control group see Brau and Woller 2004 and Armendáriz de Aghion and Morduch ( 2005 for comprehensive early surveys .