Pollen Viability of Aeschynanthus Tricolor Hook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Aeschynanthus Pulcher Lipstick Plant LIPSTICK PLANT
The Gardener’s Resource 435 W. Glenside Ave. Since 1943 Glenside, PA 19038 215-887-7500 Aeschynanthus pulcher Lipstick Plant LIPSTICK PLANT Lipstick plants are easy indoor flowering houseplants that when given the right amount of light and water, they produce numerous red or orange small tubular flowers that resemble a tube of lipstick. Not only are the flowers colorful, the leaves can be light green, dark green or green and maroon. Light: The Lipstick vine will not bloom without adequate light. They require very bright, indirect light. Avoid placing this plant in full shade or full sun. Direct sun will burn the leaves. The plant needs bright light for a portion of the day, but not all day long. Water: If you allow the top 25% of the soil to dry out before watering, this plant will flower more frequently and more abundant. If the leaves appear soft and shriveled, give it more water. These plants will also lose green leaves TIPS: when over-watered. • Lipstick plants like warm temperatures between 75-85°F. : Fertilizer every other week in the Fertilizing • Prefers high humidity, but will do well in Spring and Summer and only monthly in the basic household humidity too. Fall and Winter with a houseplant food high in • Trim the long vines to prevent the plant phosphorous. Always dilute the fertilizer to ½ from becoming thin and straggly. the recommended strength. • Lipstick Plants are Non-Poisonous. • Keep a lipstick plant in a small pot will help it produce more flowers. When put into a large container, instead of producing flowers it grows more leaves. -

Temporal and Spatial Origin of Gesneriaceae in the New World Inferred from Plastid DNA Sequences
bs_bs_banner Botanical Journal of the Linnean Society, 2013, 171, 61–79. With 3 figures Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences MATHIEU PERRET1*, ALAIN CHAUTEMS1, ANDRÉA ONOFRE DE ARAUJO2 and NICOLAS SALAMIN3,4 1Conservatoire et Jardin botaniques de la Ville de Genève, Ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland 2Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Rua Santa Adélia, 166, Bairro Bangu, Santo André, Brazil 3Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland 4Swiss Institute of Bioinformatics, Quartier Sorge, CH-1015 Lausanne, Switzerland Received 15 December 2011; revised 3 July 2012; accepted for publication 18 August 2012 Gesneriaceae are represented in the New World (NW) by a major clade (c. 1000 species) currently recognized as subfamily Gesnerioideae. Radiation of this group occurred in all biomes of tropical America and was accompanied by extensive phenotypic and ecological diversification. Here we performed phylogenetic analyses using DNA sequences from three plastid loci to reconstruct the evolutionary history of Gesnerioideae and to investigate its relationship with other lineages of Gesneriaceae and Lamiales. Our molecular data confirm the inclusion of the South Pacific Coronanthereae and the Old World (OW) monotypic genus Titanotrichum in Gesnerioideae and the sister-group relationship of this subfamily to the rest of the OW Gesneriaceae. Calceolariaceae and the NW genera Peltanthera and Sanango appeared successively sister to Gesneriaceae, whereas Cubitanthus, which has been previously assigned to Gesneriaceae, is shown to be related to Linderniaceae. Based on molecular dating and biogeographical reconstruction analyses, we suggest that ancestors of Gesneriaceae originated in South America during the Late Cretaceous. -

Ornamental Garden Plants of the Guianas Pt. 2
Surinam (Pulle, 1906). 8. Gliricidia Kunth & Endlicher Unarmed, deciduous trees and shrubs. Leaves alternate, petiolate, odd-pinnate, 1- pinnate. Inflorescence an axillary, many-flowered raceme. Flowers papilionaceous; sepals united in a cupuliform, weakly 5-toothed tube; standard petal reflexed; keel incurved, the petals united. Stamens 10; 9 united by the filaments in a tube, 1 free. Fruit dehiscent, flat, narrow; seeds numerous. 1. Gliricidia sepium (Jacquin) Kunth ex Grisebach, Abhandlungen der Akademie der Wissenschaften, Gottingen 7: 52 (1857). MADRE DE CACAO (Surinam); ACACIA DES ANTILLES (French Guiana). Tree to 9 m; branches hairy when young; poisonous. Leaves with 4-8 pairs of leaflets; leaflets elliptical, acuminate, often dark-spotted or -blotched beneath, to 7 x 3 (-4) cm. Inflorescence to 15 cm. Petals pale purplish-pink, c.1.2 cm; standard petal marked with yellow from middle to base. Fruit narrowly oblong, somewhat woody, to 15 x 1.2 cm; seeds up to 11 per fruit. Range: Mexico to South America. Grown as an ornamental in the Botanic Gardens, Georgetown, Guyana (Index Seminum, 1982) and in French Guiana (de Granville, 1985). Grown as a shade tree in Surinam (Ostendorf, 1962). In tropical America this species is often interplanted with coffee and cacao trees to shade them; it is recommended for intensified utilization as a fuelwood for the humid tropics (National Academy of Sciences, 1980; Little, 1983). 9. Pterocarpus Jacquin Unarmed, nearly evergreen trees, sometimes lianas. Leaves alternate, petiolate, odd- pinnate, 1-pinnate; leaflets alternate. Inflorescence an axillary or terminal panicle or raceme. Flowers papilionaceous; sepals united in an unequally 5-toothed tube; standard and wing petals crisped (wavy); keel petals free or nearly so. -

Diversity and Distribution of Vascular Epiphytic Flora in Sub-Temperate Forests of Darjeeling Himalaya, India
Annual Research & Review in Biology 35(5): 63-81, 2020; Article no.ARRB.57913 ISSN: 2347-565X, NLM ID: 101632869 Diversity and Distribution of Vascular Epiphytic Flora in Sub-temperate Forests of Darjeeling Himalaya, India Preshina Rai1 and Saurav Moktan1* 1Department of Botany, University of Calcutta, 35, B.C. Road, Kolkata, 700 019, West Bengal, India. Authors’ contributions This work was carried out in collaboration between both authors. Author PR conducted field study, collected data and prepared initial draft including literature searches. Author SM provided taxonomic expertise with identification and data analysis. Both authors read and approved the final manuscript. Article Information DOI: 10.9734/ARRB/2020/v35i530226 Editor(s): (1) Dr. Rishee K. Kalaria, Navsari Agricultural University, India. Reviewers: (1) Sameh Cherif, University of Carthage, Tunisia. (2) Ricardo Moreno-González, University of Göttingen, Germany. (3) Nelson Túlio Lage Pena, Universidade Federal de Viçosa, Brazil. Complete Peer review History: http://www.sdiarticle4.com/review-history/57913 Received 06 April 2020 Accepted 11 June 2020 Original Research Article Published 22 June 2020 ABSTRACT Aims: This communication deals with the diversity and distribution including host species distribution of vascular epiphytes also reflecting its phenological observations. Study Design: Random field survey was carried out in the study site to identify and record the taxa. Host species was identified and vascular epiphytes were noted. Study Site and Duration: The study was conducted in the sub-temperate forests of Darjeeling Himalaya which is a part of the eastern Himalaya hotspot. The zone extends between 1200 to 1850 m amsl representing the amalgamation of both sub-tropical and temperate vegetation. -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -
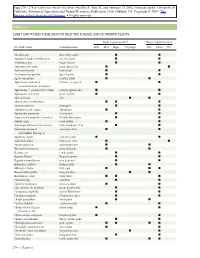
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum -

Cytotaxonomic Observations in the Genus Aeschynanthus (Gesneriaceae)
EDINB. J. BOT. 58 (1): 31–43 (2001) 31 CYTOTAXONOMIC OBSERVATIONS IN THE GENUS AESCHYNANTHUS (GESNERIACEAE) M. H. R*, K. J†‡ & M. M† This study is a contribution to the further understanding of cytological patterns in Aeschynanthus (Gesneriacaeae). Chromosome numbers are reported for 12 species from six sections; nine of these are new counts. Two basic numbers, x=16 and x=15, are generally encountered. Aeschynanthus gracilis proved to be of exceptional interest, as its rare somatic number, 2n=28, confirms the occurrence of a third basic number, x= 14, in the genus. Variation in chromosome number in relation to seed morphology is examined. Keywords. Aeschynanthus, chromosomes, cytotaxonomy, Gesneriaceae, sectional relationships. I Aeschynanthus Jack (Gesneriaceae, subfamily Cyrtandroideae, tribe Trichosporeae)is a genus of some 150 species of perennial, usually epiphytic, subshrubs distributed from India and China and throughout SE Asia to the Solomon Islands. The tribe Trichosporeae (consisting of Aeschynanthus, Agalmyla (including Dichrotrichium), Loxostigma, Lysionotus, and the doubtful monotypic genus Micraeschynanthus)is distinguished by the possession of appendages, often hair-like, at each end of the seed. The apical appendage is always single, but in Aeschynanthus the number and form of the hilar appendages are taxonomically important. Bentham (1876) first proposed a sectional classification of Aeschynanthus based almost entirely on seed appendages, and recognized four sections: Polytrichium, Diplotrichium, Haplotrichium, and Holocalyx (now sect. Aeschynanthus). Clarke (1883) added sect. Microtrichium. Schlechter (1923) added sect. Anisocalyx but this was subsumed under Microtrichium by Burtt & Woods (1975), while Wang (1984) created sect. Xanthanthos, based not on seed but on corolla characters, to accommodate a single Chinese species. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

Endemic Wild Ornamental Plants from Northwestern Yunnan, China
HORTSCIENCE 40(6):1612–1619. 2005. have played an important role in world horti- culture and have been introduced to Western countries where they have been widely cul- Endemic Wild Ornamental Plants tivated. Some of the best known examples include Rhododendron, Primula, Gentiana, from Northwestern Yunnan, China Pedicularis, and Saussurea, which are all im- 1 portant genera in northwestern Yunnan (Chen Xiao-Xian Li and Zhe-Kun Zhou et al., 1989; Feng, 1983; Guan et al., 1998; Hu, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, P.R. 1990; Shi and Jin, 1999; Yang, 1956;). Many of China 650204 these ornamental species are endemic to small areas of northwestern Yunnan (e.g., Rhododen- Additional index words. horticultural potential dron russatum), therefore, their cultivation not Abstract. Northwestern Yunnan is situated in the southern part of the Hengduan Mountains, only provides for potential sources of income which is a complex and varied natural environment. Consequently, this region supports a generation, but also offers a potential form of great diversity of endemic plants. Using fi eld investigation in combination with analysis conservation management: these plants can of relevant literature and available data, this paper presents a regional ethnobotanical be used directly for their ornamental plant study of this area. Results indicated that northwestern Yunnan has an abundance of wild value or as genetic resources for plant breed- ornamental plants: this study identifi ed 262 endemic species (belonging to 64 genera and ing programs. The aims of current paper are 28 families) with potential ornamental value. The distinguishing features of these wild to describe the unique fl ora of northwestern plants, their characteristics and habitats are analyzed; the ornamental potential of most Yunnan and provide detailed information of plants stems from their wildfl owers, but some species also have ornamental fruits and those resources, in terms of their potential foliage. -

Plant Regeneration Via Direct and Indirect Adventitious Shoot Formation and Chromosome-Doubled Somaclonal Variation in Titanotrichum Oldhamii (Hemsl.) Solereder
Plant Biotechnol Rep (2011) 5:187–195 DOI 10.1007/s11816-011-0172-5 ORIGINAL ARTICLE Plant regeneration via direct and indirect adventitious shoot formation and chromosome-doubled somaclonal variation in Titanotrichum oldhamii (Hemsl.) Solereder Hiroki Takagi • Shintaro Sugawara • Tomoka Saito • Haruka Tasaki • Lu Yuanxue • Guan Kaiyun • Dong-Sheng Han • Toshinari Godo • Masaru Nakano Received: 17 January 2011 / Accepted: 25 February 2011 / Published online: 15 March 2011 Ó Korean Society for Plant Biotechnology and Springer 2011 Abstract The gesneriaceous perennial plant Titanotri- greenhouse. Flow cytometry analysis indicated that no chum oldhamii has beautiful foliage and attractive bright variation in the ploidy level was observed in plants yellow flowers. However, breeding of T. oldhamii by regenerated via direct shoot formation, whereas chromo- conventional sexual hybridization may be difficult because some doubling occurred in several plants regenerated via sexual reproduction of this species is very rare. In the indirect shoot formation. Regenerated plants with the same present study, plant regeneration systems via both direct ploidy level as the mother plants showed almost the same and indirect formation of adventitious shoots from leaf phenotype as the mother plants, whereas chromosome- explants were established as the first step toward breeding doubled plants showed apparent morphological alterations: T. oldhamii by using biotechnological techniques. Adven- they had small and crispate flowers, and round and deep titious shoots were formed efficiently on medium con- green leaves. taining 0.1 mg l-1 benzyladenine. Histological observation showed that shoot formation on this medium occurred Keywords Flow cytometry Á Gesneriaceous plant Á directly from leaf epidermal cells without callus formation. -

Selection of Plants for Vertical Gardening and Green Roof Farming
International Research Journal of Plant and Crop Sciences ISSN: 1711-3490 Vol. 4 (3),), pp. 132-153, June, 2018. Available online at www.advancedscholarsjournals.org © Advanced Scholars Journals Review Paper SELECTION OF PLANTS FOR VERTICAL GARDENING AND GREEN ROOF FARMING Dr. A.N.Sarkar Ex-Senior Professor (International Business) & Dean (Research), Asia-Pacific Institute of Management, 3& 4 Institutional Areas, Jasola (Sarita Vihar), New Delhi E.mail: [email protected] Accepted 28 May, 2018. The choice of plant species for vertical gardens is strictly dependent on climate conditions and exposure of the wall. The plants should be light, their root system must be spreading and not of the pile form. It is also important to choose evergreen plants, so that a vertical garden will fulfill its role throughout the whole year. Life expectancy is an important factor while calculating the cost of green wall exploitation. It is recommended to choose mosses, ornamental grasses, perennials, shrubs which live a few to dozens of years with a high tolerance to environmental pollution. Aging and withered plants should be replaced during a garden maintaining process of minimum every half year. The use of different plant species including herbs, grasses, perennials and trees help to make the roof-scape a natural environment. Other factors that should be considered when selecting plants include: the rate of plant growth, nutrient needs, and sensitivity to pollution. The type of plant species and their location on the roofs also depend on the geographical location, the rate of air pollution, rooftop height, shade, growing medium depth and composition, accessibility of roof, run-off water management purposes, irrigation method, thermal insulation purposes, installation techniques and maintenance.