(Serpentes, Colubridae) from Northern Vietnam
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

ONEP V09.Pdf
Compiled by Jarujin Nabhitabhata Tanya Chan-ard Yodchaiy Chuaynkern OEPP BIODIVERSITY SERIES volume nine OFFICE OF ENVIRONMENTAL POLICY AND PLANNING MINISTRY OF SCIENCE TECHNOLOGY AND ENVIRONMENT 60/1 SOI PIBULWATTANA VII, RAMA VI RD., BANGKOK 10400 THAILAND TEL. (662) 2797180, 2714232, 2797186-9 FAX. (662) 2713226 Office of Environmental Policy and Planning 2000 NOT FOR SALE NOT FOR SALE NOT FOR SALE Compiled by Jarujin Nabhitabhata Tanya Chan-ard Yodchaiy Chuaynkern Office of Environmental Policy and Planning 2000 First published : September 2000 by Office of Environmental Policy and Planning (OEPP), Thailand. ISBN : 974–87704–3–5 This publication is financially supported by OEPP and may be reproduced in whole or in part and in any form for educational or non–profit purposes without special permission from OEPP, providing that acknowledgment of the source is made. No use of this publication may be made for resale or for any other commercial purposes. Citation : Nabhitabhata J., Chan ard T., Chuaynkern Y. 2000. Checklist of Amphibians and Reptiles in Thailand. Office of Environmental Policy and Planning, Bangkok, Thailand. Authors : Jarujin Nabhitabhata Tanya Chan–ard Yodchaiy Chuaynkern National Science Museum Available from : Biological Resources Section Natural Resources and Environmental Management Division Office of Environmental Policy and Planning Ministry of Science Technology and Environment 60/1 Rama VI Rd. Bangkok 10400 THAILAND Tel. (662) 271–3251, 279–7180, 271–4232–8 279–7186–9 ext 226, 227 Facsimile (662) 279–8088, 271–3251 Designed & Printed :Integrated Promotion Technology Co., Ltd. Tel. (662) 585–2076, 586–0837, 913–7761–2 Facsimile (662) 913–7763 2 1. -

Genus Lycodon)
Zoologica Scripta Multilocus phylogeny reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon) CAMERON D. SILER,CARL H. OLIVEROS,ANSSI SANTANEN &RAFE M. BROWN Submitted: 6 September 2012 Siler, C. D., Oliveros, C. H., Santanen, A., Brown, R. M. (2013). Multilocus phylogeny Accepted: 8 December 2012 reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon). —Zoologica doi:10.1111/zsc.12007 Scripta, 42, 262–277. The diverse group of Asian wolf snakes of the genus Lycodon represents one of many poorly understood radiations of advanced snakes in the superfamily Colubroidea. Outside of three species having previously been represented in higher-level phylogenetic analyses, nothing is known of the relationships among species in this unique, moderately diverse, group. The genus occurs widely from central to Southeast Asia, and contains both widespread species to forms that are endemic to small islands. One-third of the diversity is found in the Philippine archipelago. Both morphological similarity and highly variable diagnostic characters have contributed to confusion over species-level diversity. Additionally, the placement of the genus among genera in the subfamily Colubrinae remains uncertain, although previous studies have supported a close relationship with the genus Dinodon. In this study, we provide the first estimate of phylogenetic relationships within the genus Lycodon using a new multi- locus data set. We provide statistical tests of monophyly based on biogeographic, morpho- logical and taxonomic hypotheses. With few exceptions, we are able to reject many of these hypotheses, indicating a need for taxonomic revisions and a reconsideration of the group's biogeography. Mapping of color patterns on our preferred phylogenetic tree suggests that banded and blotched types have evolved on multiple occasions in the history of the genus, whereas the solid-color (and possibly speckled) morphotype color patterns evolved only once. -

Herp. Bulletin 101.Qxd
Mountain wolf snake ( Lycodon r . ruhstrati ) predation on an exotic lizard, Anolis sagrei , in Chiayi County, Taiwan GERRUT NORVAL 1, SHAO-CHANG HUANG 2 and JEAN-JAY MAO 3 1 Applied Behavioural Ecology & Ecosystem Research Unit, Department of Nature Conservation, UNISA, Private Bag X6, Florida, 1710, Republic of South Africa . Email: [email protected] [author for correspondence] 2 Department of Life Science, Tunghai University. No. 181, Sec. 3, Taichung-Kan Road, Taichung, 407- 04, Taiwan, R.O.C . 3 Department of Natural Resources, National I-Lan University, No. 1, Shen-Lung Rd., Sec. 1, I-Lan City 260, Taiwan, R.O.C . ABSTRACT – The Mountain wolf snake ( Lycodon ruhstrati ruhstrati ) is a common snake species at low elevations all over Taiwan. Still, it appears to be poorly studied in Taiwan and adjacent areas since little has been reported about this species. On 26 th August 2002 ten L. r. ruhstrati eggs were obtained from an adult female, one of two that were caught a day before, and eight of the eggs hatched successfully on 14 th October 2002. While in captivity all the adults preyed upon Anolis sagrei , which were given to them as prey, while two neonates accepted A. sagrei hatchlings offered to them as food. On February 18 th , 2006, a DOR Mountain wolf snake, with an A. sagrei in its stomach, was found on a tarred road in Santzepu, Sheishan District, Chiayi County. This appears to be the first report from Taiwan of the Mountain wolf snake ( L. r. ruhstrati ) preying on the exotic introduced lizard A. -

New Country Records of Reptiles from Laos
Biodiversity Data Journal 1: e1015 doi: 10.3897/BDJ.1.e1015 Taxonomic paper New country records of reptiles from Laos Vinh Quang Luu†,‡, Truong Quang Nguyen§,|, Thomas Calame¶, Tuoi Thi Hoang#, Sisomphone Southichack††, Michael Bonkowski|, Thomas Ziegler‡,| † Department of Wildlife, Faculty of Natural Resource and Environmental Management, Vietnam Forestry University, Xuan Mai, Chuong My, Hanoi, Vietnam ‡ AG Zoologischer Garten Köln, Riehler Strasse 173, D-50735 Cologne, Germany § Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Vietnam | Zoological Institute, University of Cologne, Zülpicher Strasse 47b, D-50674 Cologne, Germany ¶ WWF Greater Mekong, House No. 39, Unit 05, Ban Saylom, Vientiane, Lao People's Democratic Republic # Biodiversity Center, Faculty of Natural Resource and Environmental Management, Vietnam Forestry University, Xuan Mai, Chuong My, Hanoi, Vietnam †† Hin Nam No National Protected Area, Boualapha District, Khammouane Province, Lao People's Democratic Republic Corresponding author: Vinh Quang Luu ([email protected]) Academic editor: Johannes Penner Received: 27 Oct 2013 | Accepted: 04 Dec 2013 | Published: 10 Dec 2013 Citation: Luu V, Nguyen T, Calame T, Hoang T, Southichack S, Bonkowski M, Ziegler T (2013) New country records of reptiles from Laos. Biodiversity Data Journal 1: e1015. doi: 10.3897/BDJ.1.e1015 Abstract Four species of reptiles, of which one is represented by one of its subspecies, are recorded for the first time from Laos: Cyrtodactylus phongnhakebangensis, Lycodon futsingensis, and L. ruhstrati, as L. ruhstrati abditus, from limestone forests in Khammouane Province and Cyrtodactylus pseudoquadrivirgatus from hill evergreen forest in Salavan Province. These discoveries of lizards and snakes bring the total species number of reptiles to 189 in Laos. -
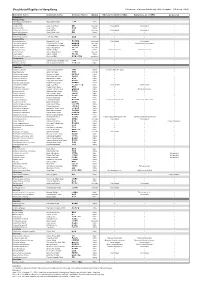
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

P. 1 AC27 Inf. 7 (English Only / Únicamente En Inglés / Seulement
AC27 Inf. 7 (English only / únicamente en inglés / seulement en anglais) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Species trade and conservation IUCN RED LIST ASSESSMENTS OF ASIAN SNAKE SPECIES [DECISION 16.104] 1. The attached information document has been submitted by IUCN (International Union for Conservation of * Nature) . It related to agenda item 19. * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat or the United Nations Environment Programme concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. AC27 Inf. 7 – p. 1 Global Species Programme Tel. +44 (0) 1223 277 966 219c Huntingdon Road Fax +44 (0) 1223 277 845 Cambridge CB3 ODL www.iucn.org United Kingdom IUCN Red List assessments of Asian snake species [Decision 16.104] 1. Introduction 2 2. Summary of published IUCN Red List assessments 3 a. Threats 3 b. Use and Trade 5 c. Overlap between international trade and intentional use being a threat 7 3. Further details on species for which international trade is a potential concern 8 a. Species accounts of threatened and Near Threatened species 8 i. Euprepiophis perlacea – Sichuan Rat Snake 9 ii. Orthriophis moellendorfi – Moellendorff's Trinket Snake 9 iii. Bungarus slowinskii – Red River Krait 10 iv. Laticauda semifasciata – Chinese Sea Snake 10 v. -

(GISD) 2021. Species Profile Norops Sagrei. Available From
FULL ACCOUNT FOR: Norops sagrei Norops sagrei System: Terrestrial Kingdom Phylum Class Order Family Animalia Chordata Reptilia Squamata Polychrotidae Common name Bahamian brown anole (English), Cuban brown anole (English), brown anole (English) Synonym Anolis sagrei , (Cocteau in Dum?ril and Bibron, 1837) Similar species Anolis carolinensis Summary Norops sagrei (brown anole) can be identified by its extensible throat fan that is often coloured yellow or reddish-orange and has a white line down the centre of its back. Norops sagrei is a habitat generalist that prefers the open vegetation of disturbed sites. It is a ground dweller but will venture several feet up into trees and shrubs. Norops sagrei compete with Anolis carolinensis and other introduced congeners. Norops sagre also prey on the hatchlings of Anolis carolinensis. view this species on IUCN Red List Species Description Norops sagrei (brown anole) is a “trunk ground ecomorph” sensu (Williams, 1983). It is described as having an extensible throat fan that can be yellow to red-orange. This species can be between 13 and 21.3cm. It also has enlarged toe pads and a short snout (Campbell, 2002). Brown anoles can erect a dorsonuchal crest when exposed to certain stimuli. The tail may have a crest-like ridge, but this is highly variable between individuals and should not be confused with the dorsonuchal crest. Also, the tail is laterally compressed. Females have a light line down the middle of their backs, but males do not. They tend to have a lighter mid-dorsal stripe that is distinct and often boldly patterned in females and often indistinct in males. -

List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Cheloniidae 4 Loggerhead Turtle Caretta caretta Green Turtle Chelonia mydas Hawksbill Turtle Eretmochelys imbricata Olive Ridley Turtle Lepidochelys olivacea Dermochelyidae 1 Leatherback Turtle Dermochelys coriacea Emydidae 1 Red-eared Slider * Trachemys scripta elegans Geoemydidae 3 Three-banded Box Turtle Cuora trifasciata Reeves' Turtle Mauremys reevesii Beale's Turtle Sacalia bealei Platysternidae 1 Big-headed Turtle Platysternon megacephalum Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Agamidae 1 Changeable Lizard Calotes versicolor Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Long-tailed Skink Eutropis longicaudata Chinese Skink Plestiodon chinensis chinensis Five-striped Blue-tailed Skink Plestiodon elegans Blue-tailed Skink Plestiodon quadrilineatus Vietnamese Five-lined Skink Plestiodon tamdaoensis Slender Forest Skink Scincella modesta Reeve's Smooth Skink Scincella reevesii Brown Forest Skink Sphenomorphus incognitus Indian Forest Skink Sphenomorphus indicus Chinese Waterside Skink Tropidophorus sinicus Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek's Burrowing Lizard Dibamus bogadeki Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko Gekko gecko Bowring's Gecko Hemidactylus bowringii Brook's Gecko* Hemidactylus brookii House Gecko* Hemidactylus -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -

New Records and an Updated Checklist of Amphibians and Snakes From
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Bonn zoological Bulletin - früher Bonner Zoologische Beiträge. Jahr/Year: 2021 Band/Volume: 70 Autor(en)/Author(s): Le Dzung Trung, Luong Anh Mai, Pham Cuong The, Phan Tien Quang, Nguyen Son Lan Hung, Ziegler Thomas, Nguyen Truong Quang Artikel/Article: New records and an updated checklist of amphibians and snakes from Tuyen Quang Province, Vietnam 201-219 Bonn zoological Bulletin 70 (1): 201–219 ISSN 2190–7307 2021 · Le D.T. et al. http://www.zoologicalbulletin.de https://doi.org/10.20363/BZB-2021.70.1.201 Research article urn:lsid:zoobank.org:pub:1DF3ECBF-A4B1-4C05-BC76-1E3C772B4637 New records and an updated checklist of amphibians and snakes from Tuyen Quang Province, Vietnam Dzung Trung Le1, Anh Mai Luong2, Cuong The Pham3, Tien Quang Phan4, Son Lan Hung Nguyen5, Thomas Ziegler6 & Truong Quang Nguyen7, * 1 Ministry of Education and Training, 35 Dai Co Viet Road, Hanoi, Vietnam 2, 5 Hanoi National University of Education, 136 Xuan Thuy Road, Hanoi, Vietnam 2, 3, 7 Institute of Ecology and Biological Resources, Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 6 AG Zoologischer Garten Köln, Riehler Strasse 173, D-50735 Köln, Germany 6 Institut für Zoologie, Universität Köln, Zülpicher Strasse 47b, D-50674 Köln, Germany * Corresponding author: Email: [email protected] 1 urn:lsid:zoobank.org:author:2C2D01BA-E10E-48C5-AE7B-FB8170B2C7D1 2 urn:lsid:zoobank.org:author:8F25F198-A0F3-4F30-BE42-9AF3A44E890A 3 urn:lsid:zoobank.org:author:24C187A9-8D67-4D0E-A171-1885A25B62D7 4 urn:lsid:zoobank.org:author:555DF82E-F461-4EBC-82FA-FFDABE3BFFF2 5 urn:lsid:zoobank.org:author:7163AA50-6253-46B7-9536-DE7F8D81A14C 6 urn:lsid:zoobank.org:author:5716DB92-5FF8-4776-ACC5-BF6FA8C2E1BB 7 urn:lsid:zoobank.org:author:822872A6-1C40-461F-AA0B-6A20EE06ADBA Abstract. -

Reptiles of Phetchaburi Province, Western Thailand: a List of Species, with Natural History Notes, and a Discussion on the Biogeography at the Isthmus of Kra
The Natural History Journal of Chulalongkorn University 3(1): 23-53, April 2003 ©2003 by Chulalongkorn University Reptiles of Phetchaburi Province, Western Thailand: a list of species, with natural history notes, and a discussion on the biogeography at the Isthmus of Kra OLIVIER S.G. PAUWELS 1*, PATRICK DAVID 2, CHUCHEEP CHIMSUNCHART 3 AND KUMTHORN THIRAKHUPT 4 1 Department of Recent Vertebrates, Institut Royal des Sciences naturelles de Belgique, 29 rue Vautier, 1000 Brussels, BELGIUM 2 UMS 602 Taxinomie-collection – Reptiles & Amphibiens, Muséum National d’Histoire Naturelle, 25 rue Cuvier, 75005 Paris, FRANCE 3 65 Moo 1, Tumbon Tumlu, Amphoe Ban Lat, Phetchaburi 76150, THAILAND 4 Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, THAILAND ABSTRACT.–A study of herpetological biodiversity was conducted in Phetcha- buri Province, in the upper part of peninsular Thailand. On the basis of a review of available literature, original field observations and examination of museum collections, a preliminary list of 81 species (12 chelonians, 2 crocodiles, 23 lizards, and 44 snakes) is established, of which 52 (64 %) are reported from the province for the first time. The possible presence of additional species is discussed. Some biological data on the new specimens are provided including some range extensions and new size records. The herpetofauna of Phetchaburi shows strong Sundaic affinities, with about 88 % of the recorded species being also found south of the Isthmus of Kra. A biogeographic affinity analysis suggests that the Isthmus of Kra plays the role of a biogeographic filter, due both to the repeated changes in climate during the Quaternary and to the current increase of the dry season duration along the peninsula from south to north. -

Article (Published Version)
Article Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world BOLON, Isabelle, et al. Abstract Background Snakebite envenoming is a major global health problem that kills or disables half a million people in the world’s poorest countries. Biting snake identification is key to understanding snakebite eco-epidemiology and optimizing its clinical management. The role of snakebite victims and healthcare providers in biting snake identification has not been studied globally. Objective This scoping review aims to identify and characterize the practices in biting snake identification across the globe. Methods Epidemiological studies of snakebite in humans that provide information on biting snake identification were systematically searched in Web of Science and Pubmed from inception to 2nd February 2019. This search was further extended by snowball search, hand searching literature reviews, and using Google Scholar. Two independent reviewers screened publications and charted the data. Results We analysed 150 publications reporting 33,827 snakebite cases across 35 countries. On average 70% of victims/bystanders spotted the snake responsible for the bite and 38% captured/killed it and brought it to the healthcare facility. [...] Reference BOLON, Isabelle, et al. Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world. PLOS ONE, 2020, vol. 15, no. 3, p. e0229989 PMID : 32134964 DOI : 10.1371/journal.pone.0229989 Available at: http://archive-ouverte.unige.ch/unige:132992 Disclaimer: layout of this document may differ from the published version. 1 / 1 PLOS ONE RESEARCH ARTICLE Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world 1 1 1 1,2 Isabelle BolonID *, Andrew M.