Phase Transformation of Oxide Scale and Its Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metals and Metal Products Tariff Schedules of the United States
251 SCHEDULE 6. - METALS AND METAL PRODUCTS TARIFF SCHEDULES OF THE UNITED STATES SCHEDULE 6. - METALS AND METAL PRODUCTS 252 Part 1 - Metal-Bearing Ores and Other Metal-Bearing Schedule 6 headnotes: Materials 1, This schedule does not cover — Part 2 Metals, Their Alloys, and Their Basic Shapes and Forms (II chemical elements (except thorium and uranium) and isotopes which are usefully radioactive (see A. Precious Metals part I3B of schedule 4); B. Iron or Steel (II) the alkali metals. I.e., cesium, lithium, potas C. Copper sium, rubidium, and sodium (see part 2A of sched D. Aluminum ule 4); or E. Nickel (lii) certain articles and parts thereof, of metal, F. Tin provided for in schedule 7 and elsewhere. G. Lead 2. For the purposes of the tariff schedules, unless the H. Zinc context requires otherwise — J. Beryllium, Columbium, Germanium, Hafnium, (a) the term "precious metal" embraces gold, silver, Indium, Magnesium, Molybdenum, Rhenium, platinum and other metals of the platinum group (iridium, Tantalum, Titanium, Tungsten, Uranium, osmium, palladium, rhodium, and ruthenium), and precious- and Zirconium metaI a Iloys; K, Other Base Metals (b) the term "base metal" embraces aluminum, antimony, arsenic, barium, beryllium, bismuth, boron, cadmium, calcium, chromium, cobalt, columbium, copper, gallium, germanium, Part 3 Metal Products hafnium, indium, iron, lead, magnesium, manganese, mercury, A. Metallic Containers molybdenum, nickel, rhenium, the rare-earth metals (Including B. Wire Cordage; Wire Screen, Netting and scandium and yttrium), selenium, silicon, strontium, tantalum, Fencing; Bale Ties tellurium, thallium, thorium, tin, titanium, tungsten, urani C. Metal Leaf and FoU; Metallics um, vanadium, zinc, and zirconium, and base-metal alloys; D, Nails, Screws, Bolts, and Other Fasteners; (c) the term "meta I" embraces precious metals, base Locks, Builders' Hardware; Furniture, metals, and their alloys; and Luggage, and Saddlery Hardware (d) in determining which of two or more equally specific provisions for articles "of iron or steel", "of copper", E. -

Section XV BASE METALS and ARTICLES of BASE METAL Notes
Section XV BASE METALS AND ARTICLES OF BASE METAL Notes. 1.- This Section does not cover : (a) Prepared paints, inks or other products with a basis of metallic flakes or powder (headings 32.07 to 32.10, 32.12, 32.13 or 32.15); (b) Ferro-cerium or other pyrophoric alloys (heading 36.06); (c) Headgear or parts thereof of heading 65.06 or 65.07; (d) Umbrella frames or other articles of heading 66.03; (e) Goods of Chapter 71 (for example, precious metal alloys, base metal clad with precious metal, imitation jewellery); (f) Articles of Section XVI (machinery, mechanical appliances and electrical goods); (g) Assembled railway or tramway track (heading 86.08) or other articles of Section XVII (vehicles, ships and boats, aircraft); (h) Instruments or apparatus of Section XVIII, including clock or watch springs; (ij) Lead shot prepared for ammunition (heading 93.06) or other articles of Section XIX (arms and ammunition); (k) Articles of Chapter 94 (for example, furniture, mattress supports, lamps and lighting fittings, illuminated signs, prefabricated buildings); (l) Articles of Chapter 95 (for example, toys, games, sports requisites); (m) Hand sieves, buttons, pens, pencil-holders, pen nibs or other articles of Chapter 96 (miscellaneous manufactured articles); or (n) Articles of Chapter 97 (for example, works of art). 2.- Throughout the Nomenclature, the expression “parts of general use” means : (a) Articles of heading 73.07, 73.12, 73.15, 73.17 or 73.18 and similar articles of other base metal; (b) Springs and leaves for springs, of base metal, other than clock or watch springs (heading 91.14); and (c) Articles of headings 83.01, 83.02, 83.08, 83.10 and frames and mirrors, of base metal, of heading 83.06. -

GLOSSARY of PLATING TERMS Acid Gold: a Mildly Acidic Process That Is Used When Plating from 7 to 200 Mils of Gold
GLOSSARY OF PLATING TERMS Acid gold: A mildly acidic process that is used when plating from 7 to 200 mils of gold. The deposits are usually 23kt purity, and it is not usually used as the final finish. Antique: A process which involves the application of a dark top coating over bronze or silver. This coating, either plated or painted, is partially removed to expose some of the underlying metal. Anti-tarnish: A protective coating that provides minimal tarnish protection for a low cost. Barrel plating: A type of mass finishing that takes place in a barrel or tub. Barrel plating is usually requested for very small pieces where pricing must be kept low. Black nickel: A bright or matte, dark plating process that is used to highlight antique finishes. Or, when used as a final color it will range from dark grey to light black. A bright black nickel will yield the darkest color. Bright finish: A high luster, smooth finish. CASS testing: The Copper-Accelerated Acetic Acid Salt Spray test is the same as the Neutral Salt Spray (NSS) test, except it is accelerated, with typical time cycles being 8 and 24 hours. Cold nickel: A non-brightened nickel bath which replicates the original finish, that is, bright areas remain bright and dull areas remain dull. Color: Describes the final top coating (flash) which could be white, silver, 14kt gold (Hamilton), 18kt gold, or 24kt gold (English gold). See "gold flash" and "cyanide gold." Copper: An excellent undercoat in the plating process. Copper provides good conductivity and forms an excellent protective barrier between the base metal and the plate. -

The Care and Preservation of Historical Silver by CLARA DECK, CONSERVATOR REVISIONS by LOUISE BECK, CONSERVATOR
The Care and Preservation of Historical Silver BY CLARA DECK, CONSERVATOR REVISIONS BY LOUISE BECK, CONSERVATOR Introduction Historical silver can be maintained for years of use and enjoyment provided that some basic care and attention is given to their preservation. The conservation staff at The Henry Ford have compiled the information in this fact sheet to help individuals care for their objects and collections. The first step in the care of all collections is to understand and minimize or eliminate conditions that can cause damage. The second step is to follow basic guidelines for care, handling and cleaning. Most people know that silver is a white, lustrous metal. Pure or “fine” silver is called “Sterling” if it is made up of no less than 925 parts silver to 75 parts alloy. Sterling will thus often have ‘.925’ stamped somewhere on it, as an identifier. Silver objects, especially coins and jewelry, contain copper as an alloying metal for added hardness. The copper may corrode to form dark brown or green deposits on the surface of the metal. Silver is usually easy to differentiate from lead or pewter, which are generally dark gray and not very shiny. Silver is often plated (deposited) onto other metallic alloys, almost always with an intermediate layer of copper in between. The earliest plating process, “Sheffield Plate” was developed in England in 1742. By the mid-19th century, the process was largely replaced by electroplating (which used less silver). The base metal in plated artifacts may consist of any of the following metals or alloys: copper, brass, “German silver” or “nickel silver” (50% copper, 30% nickel, 20% zinc), “Brittania metal” (97% tin, 7% antimony, 2% copper), or a “base” silver containing a high percentage of copper. -

A Decomposition Analysis of Base Metal Prices: Comparing the Effect of Detrending Methods on Trend Identification and Cyclical Components
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Dissertations, Master's Theses and Master's Reports - Open Reports 2013 A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS Mahmoodreza Modirroosta Michigan Technological University Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Economics Commons Copyright 2013 Mahmoodreza Modirroosta Recommended Citation Modirroosta, Mahmoodreza, "A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS", Master's Thesis, Michigan Technological University, 2013. https://doi.org/10.37099/mtu.dc.etds/674 Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Economics Commons A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS By Mahmoodreza Modirroosta A THESIS Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Applied Natural Resource Economics MICHIGAN TECHNOLOGICAL UNIVERSITY 2013 © Mahmoodreza Modirroosta This thesis has been approved in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE in Applied Natural Resource Economics School of Business and Economics Thesis Advisor: Dr. Mark Roberts Committee Member: Dr. Xavier de Castro Oliveira Committee Member: Dr. Seyyedmohsen -
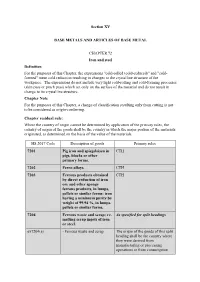
Section XV BASE METALS and ARTICLES of BASE METAL
Section XV BASE METALS AND ARTICLES OF BASE METAL CHAPTER 72 Iron and steel Definition For the purposes of this Chapter, the expressions "cold-rolled (cold-reduced)" and "cold- formed" mean cold reduction resulting in changes to the crystalline structure of the workpiece. The expressions do not include very light cold-rolling and cold-forming processes (skin pass or pinch pass) which act only on the surface of the material and do not result in change to its crystalline structure. Chapter Note For the purposes of this Chapter, a change of classification resulting only from cutting is not to be considered as origin-conferring. Chapter residual rule: Where the country of origin cannot be determined by application of the primary rules, the country of origin of the goods shall be the country in which the major portion of the materials originated, as determined on the basis of the value of the materials. HS 2017 Code Description of goods Primary rules 7201 Pig iron and spiegeleisen in CTH pigs, blocks or other primary forms. 7202 Ferro-alloys. CTH 7203 Ferrous products obtained CTH by direct reduction of iron ore and other spongy ferrous products, in lumps, pellets or similar forms; iron having a minimum purity by weight of 99.94 %, in lumps, pellets or similar forms. 7204 Ferrous waste and scrap; re- As specified for split headings melting scrap ingots of iron or steel. ex7204(a) - Ferrous waste and scrap The origin of the goods of this split heading shall be the country where they were derived from manufacturing or processing operations or from consumption HS 2017 Code Description of goods Primary rules ex7204(b) - Re-melting scrap ingots of The origin of the goods of this split iron or steel heading shall be the country where the waste and scrap used to obtain them were derived from manufacturing or processing operations or from consumption 7205 Granules and powders, of As specified for subheadings pig iron, spiegeleisen, iron or steel. -

Procedure 12751-MNGP-OPS-01, Revision 0, Spent Fuel Cask Welding
ENCLOSURE 6 PROCEDURE 12751-MNGP-OPS-01, REVISION 0 SPENT FUEL CASK WELDING: 61 BT/BTH NU HOMS CANISTERS 29 pages follow Spent Fuel Cask Welding: 61BT/BTH NUHOMS Canisters DSC#: " Owner Approval: _____ I)ate: ~~~ Owner Approval: _____ I)ate: ~~~ Page 1 ofl9 Spent Fuel Cask Weldlng-61BT/BTH NUHOMS Canisters 127Sl~MNGP-OPS-OI, REV 0 REVISION SUMMARY Rev O Initial Draft Version: Drafted fran TrlVls Core document (NIIS 3 R7. Modified attachments 9.2 and 9.3 per NUH61BTH-4008, NUHOMS 61BTH TYPE 1 & 2 TRANSPORTABLE CANISTER FOR BWR FUEL FIELD WELDING. Approved By Date , Reviewed By Date c"' (.1>4/t 1 /2013> Approved By Date 4\t\\ur~ Page 2 of2!> Spent Fuel Cask Welding-61BT/BTH NUHOMS Canisters 12751-MNGP-OPS-OI, REV 0 TABLE OF CONTENTS SECTION PAQE 1.0 PURPOSE 4 2.0 APPLICABILITY 4 3.0 REFERENCES 4 4.0 REQUIREMENTS 4 s.o DEFINITIONS 4 6.0 RESPONSIBILITIES 4 6.1 Welding Supervisor Duties and Responsibilities 4 6.2 Job Supervisor Duties and Responsibilities 5 6.3 Welder Duties and Responsibilities 5 7.0 SPECIAL INSTRUCTIONS 5 8.0 PROCEDURE 6 8. t General GuldeUnes 6 8.2 GTAW Restriction 6 8.3 Preparation of Base Materials 6 8.4 Assembly 7 8.5 Preheat 8 8.6 Alignment 8 8.7 lnterpass 9 8.8 Rework of In-process Welds 9 8.9 Weld and HAZ Conditions Prior to NDE 9 8.10 Repair of Weld Metal and HAZ Defects Post NDE 10 8. t 1 Base Metal Repairs 11 8.12 Records 11 9.0 A ITACHMENTS 12 9.1 Terms and Definitions 13-16 9.2 Weld Map 17-19 9.3 Weld Data Sheet 20-.25 9.4 Fit up / High-Low / Depth Record 26 9.5 Field Comment and/or Repair Log 27-29 Page3of29 Spent Fuel Cask Welding-61BT/BTH NUHOMS Canisters 12751-MNGP-OPS-Ol, REV 0 1.0 PURPOSE 1.1 Provides detailed instructions for making welds on the NUHOMS spent fuel cask canisters. -

Castable Metal Alloys in Dentistry
- Denta y l Le em a d rn a in c g A & O ing SHA Train Castable Metal Alloys in Dentistry Martin S. Spiller, DMD Edited by Michelle Jameson, MA, Health Science Editor August 2011 A two-hour home study course Academy - Dental Learning and OSHA Training 1510 Mainstreet - Suite 420 Hopkins, MN 55343 Tel: 800-522-1207 [email protected] www.DentalLearning.org The Academy - Dental Learning and OSHA Training is an ADA CERP Recognized Provider. ADA CERP is a service of the American Dental Association to assist dental professionals in identifying quality providers of continuing dental education. ADA CERP does not approve or endorse individual courses or instructors, nor does it imply acceptance of credit hours by boards of dentistry. Concerns or complaints about a CE provider may be directed to the provider or to ADA CERP at www.ada.org/goto/cerp. Castable Metal Alloys in Dentistry Table of Contents Table of Contents 4 Course Outline, Learning Objectives 5 Martin S. Spiller DMD 5 Introduction 5 The History and Description of the Lost Wax Technique in Dentistry 6 Solids, Liquids and the Chemistry of Metals 10 Strengthening Soft Metal Structures 14 Porcelain Alloys 17 Composition of Porcelain Alloys 21 Metals and Their Uses in Dental Alloys 24 Conclusion 27 References 28 4 Castable Metal Alloys in Dentistry Course Description Castable Metal Alloys in Dentistry is a course that gives you everything you really need to know (and just about everything you ever wanted to ask) about wrought and castabl e metal dental alloys. What is the difference between type I, II, and III gold? What is palladium, and how does it affect the alloy? How about all t he other trace metals in an alloy? How does porcelain stick to a metallic substructure? Why choose o ne type of metal for a removable partial denture fr amework as opposed to another? What's the difference between grains and c rystals? Why is gold soft? What is "strain hardening" and "cold working"? Who is allergic to which metals? We make it simple and interesting. -

Hallmarking Guidance Notes
HALLMARKING GUIDANCE NOTES PRACTICAL GUIDANCE IN RELATION TO THE HALLMARKING ACT 1973 INFORMATION FROM THE ASSAY OFFICES OF GREAT BRITAIN OCTOBER 2016 London Edinburgh Birmingham Sheffield Guaranteeing The Quality Of Precious Metals Since 1327 HALLMARKING GUIDANCE NOTES HALLMARKING GUIDANCE NOTES THE PURPOSE OF THESE HALLMARKING PRECIOUS METALS GUIDANCE NOTES WHY ARE PRECIOUS METAL ARTICLES The purpose of these notes is to give practical guidance in relation to the HALLMARKED? Hallmarking Act 1973 and subsequent amendments. No reliance must be placed on the document for a legal interpretation. The UK Assay Offices are happy to Silver, palladium, gold and platinum are rarely used in their purest form but answer questions arising from these guidance notes and on any articles or other instead they are normally alloyed with lesser metals in order to achieve a issues not specifically mentioned. desired strength, durability, colour etc. It is not possible to detect by sight or by touch the gold, silver, platinum or palladium content of an item. It is therefore a legal requirement to hallmark CONTENTS OF THIS BOOKLET: all articles consisting of silver, palladium, gold or platinum (subject to certain exemptions) if they are to be described as such. Contents Page The main offence under the UK Hallmarking Act 1973 is based on description. It is Hallmarking precious metals 3 - 17 an offence for any person in the course of trade or business to: Guidance on describing precious metals 18 - 19 • Describe an un-hallmarked article as being wholly or partly made of silver, palladium, gold or platinum. Contact details for UK Assay Offices Back Page • Supply or offer to supply un-hallmarked articles to which such a description is applied. -

Hts Section Xv Base Metals and Articles of Base Metal
Harmonized Tariff Schedule of the United States (2010) Annotated for Statistical Reporting Purposes SECTION XV BASE METALS AND ARTICLES OF BASE METAL XV-1 Notes 1. This section does not cover: (a) Prepared paints, inks or other products with a basis of metallic flakes or powder (headings 3207 to 3210, 3212, 3213 or 3215); (b) Ferrocerium or other pyrophoric alloys (heading 3606); (c) Headgear or parts thereof of heading 6506 or 6507; (d) Umbrella frames or other articles of heading 6603; (e) Goods of chapter 71 (for example, precious metal alloys, base metal clad with precious metal, imitation jewelry); (f) Articles of section XVI (machinery, mechanical appliances and electrical goods); (g) Assembled railway or tramway track (heading 8608) or other articles of section XVII (vehicles, ships and boats, aircraft); (h) Instruments or apparatus of section XVIII, including clock or watch springs; (ij) Lead shot prepared for ammunition (heading 9306) or other articles of section XIX (arms and ammunition); (k) Articles of chapter 94 (for example, furniture, mattress supports, lamps and lighting fittings, illuminated signs, prefabricated buildings); (l) Articles of chapter 95 (for example, toys, games, sports equipment); (m) Hand sieves, buttons, pens, pencil-holders, pen nibs or other articles of chapter 96 (miscellaneous manufactured articles); or (n) Articles of chapter 97 (for example, works of art). 2. Throughout the tariff schedule, the expression "parts of general use" means: (a) Articles of heading 7307, 7312, 7315, 7317 or 7318 and similar articles of other base metals; (b) Springs and leaves for springs, of base metal, other than clock or watch springs (heading 9114); and (c) Articles of heading 8301, 8302, 8308 or 8310 and frames and mirrors, of base metal, of heading 8306. -

University of Nevada, Reno Gold and Base Metal Mineralization
University of Nevada, Reno Gold and Base Metal Mineralization, Hydrothermal Alteration and Vein Paragenesis in the Spring Valley Deposit, Pershing County, Nevada A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Geology by Betsy L. Crosby Dr. Tommy B. Thompson/Thesis Advisor December, 2012 THE GRADUATE SCHOOL We recommend that the thesis prepared under our supervision by BETSY CROSBY entitled Gold And Base Metal Mineralization, Hydrothermal Alteration And Vein Paragenesis In The Spring Valley Deposit, Pershing County, Nevada be accepted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Tommy B. Thompson, Ph.D., Advisor Peter Vikre, Ph.D., Committee Member Victor Vasquez, Ph.D., Graduate School Representative Marsha H. Read, Ph. D., Dean, Graduate School December, 2012 i Abstract Spring Valley is a gold-base metal deposit located on the eastern flank of the Humboldt Range in Pershing County, Nevada. Mineralization is primarily hosted within the Feldspar Porphyry, Agglomerate, Welded Tuff, Volcaniclastic Siltstone and Quartz- eye Rhyolite units of the Rochester Formation and in lithic tuffs and greenstones of the Limerick Formation. It occurs in a series of crosscutting quartz-carbonate ± pyrite ± tourmaline ± sphalerite ± galena veins of clear mesozonal origin associated with weakly to strongly pervasive quartz-sericite-pyrite-carbonate ± tourmaline alteration. Initial classification as a porphyry gold system is thus disproved, and alternate orogenic and reduced intrusion-related gold interpretations are presented. Vein-related alteration is relatively consistent throughout the Quartz-eye Rhyolite, Welded Tuff, Agglomerate and Feldspar Porphyry members of the Rochester Formation, though it is better developed in the latter. -

Rrfew Simple Techniques, Findings
Rfew simple techniques, findings, and tools are all you need to put together rand finish the projects in this book. Once you are familiar with them, it will be easier to concentrate on the creative aspects of designing a piece, and you will have the confidence you need to \ begin a great project. findings Finishing a piece of jewelry is sometimes the most important step in the process of creating it. You want a piece that will hold up and look professional at the same time. The right findings—^the metal components that hold a piece of jew- elry together—can really enhance a piece. Choose findings that complement the other materials in the piece and don't detract from the overall look you are trying to achieve. Bead shops, craft stores, and catalogs sell a variety of findings for making jewelry. The findings listed here are commonly used in jewelrymaking. JUMP RINGS are small piece to attach purchased metal loops that are used to ear wires. link components together. Jump rings are available in HEAD PINS are short fine or base metal. wires with a ball or flat pad on one end. You can hang a SPLIT RINGS are like bead on a head pin and key rings. You can use then coil the pin's top to them in place of iump rings make a loop for hanging. without having to use pli- Open jump rings by twisting the ers. They are great for EYE PINS are similar to ends out to each side with pliers. heavy pieces or stressed head pins, except they have Close jump rings in the same fash- areas where you don't want a loop instead of a ball at the ion by bringing the ends back in from the sides.