Castable Metal Alloys in Dentistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cast Stainless Steel Technology Developments
Cast Stainless Steel Technology Developments Raymond Monroe SFSA CN3MN CF8 CD4Cu CB 7 Cu CA 15 Calculation of Chromium Equivalent and Nickel Equivalent CrE = %Cr + 2 × %Si + 1.5 × %Mo + 5 × %V NiE = %Ni + 0.5 × %Mn + 30 × %C + 0.3 × %Cu Ferrite from Chemistry Schoefer Diagram 2.2 2.1 • Chemistry: C=0.07, 2 Mn=0.56, Si=1.30, 1.9 P=0.028, S=0.009, 1.8 o i t 1.7 Cr=19.5, Ni=10.7, a 1.6 on R i t i Mo=2.18 (Cb ~ 0.05 1.5 pos m o and N ~ 0.04) 1.4 C i N / r 1.3 C • ASTM A800 predicts 1.2 10.5 volume percent 1.1 1 ferrite with a range of Cr Cr (%) + 1.5Si (%) + 1.4 Mo (%) + Nb (%) − 4.99 0.9 e = 6.5 to 14.5 (chromium Ni e Ni (%) + 30 C (%) + 0.5Mn (%) + 26 ( N − 0.02 %) + 2.77 0.8 0 10203040506070 equivalent to nickel Volume Percent Ferrite equivalent = 1.19) Means of Calculating Ferrite • Severn Gage: 11 • Feritscope: 7 • Magne-Gage: 2 • Two different instruments: 5 • Manual point count • ASTM A800 • 1949 Schaeffler Diagram • WRC Diagram Unit of measure: • FN: 8 (all 4 of the non- foundry) • Volume percent: 7 • Use both methods: 2 Identification of Phases by Composition FERRITE AUSTENITE Stainless Steels - Strength Grade Yield (ksi) UTS (ksi) CF8 70 30 CF3MN 75 37 4A(2205) 90 60 6A(Zeron 100) 100 65 Stainless Steel - Corrosion Grade Critical pitting temperature oC CF8 5 (calculated) CF3MN 29 (calculated) 4A(2205) 35 - 40 6A(Zeron100) 45 – 55 Pseudo Phase Diagram for 68 % Fe – Cr Ni CCT Diagram - CD3MN 5oC/min 2oC/min 1oC/min 0.5oC/min 0.1oC/min 0.01oC/min 1100 1050 TTT curves 1000 (initial & final) 950 900 850 800 750 700 Temp. -

Repoussé Work for Amateurs
rf Bi oN? ^ ^ iTION av op OCT i 3 f943 2 MAY 8 1933 DEC 3 1938 MAY 6 id i 28 dec j o m? Digitized by the Internet Archive in 2011 with funding from Boston Public Library http://www.archive.org/details/repoussworkforamOOhasl GROUP OF LEAVES. Repousse Work for Amateurs. : REPOUSSE WORK FOR AMATEURS: BEING THE ART OF ORNAMENTING THIN METAL WITH RAISED FIGURES. tfjLd*- 6 By L. L. HASLOPE. ILLUSTRATED. LONDON L. UPCOTT GILL, 170, STRAND, W.C, 1887. PRINTED BY A. BRADLEY, 170, STRAND, LONDON. 3W PREFACE. " JjJjtfN these days, when of making books there is no end," ^*^ and every description of work, whether professional or amateur, has a literature of its own, it is strange that scarcely anything should have been written on the fascinating arts of Chasing and Repousse Work. It is true that a few articles have appeared in various periodicals on the subject, but with scarcely an exception they treated only of Working on Wood, and the directions given were generally crude and imperfect. This is the more surprising when we consider how fashionable Repousse Work has become of late years, both here and in America; indeed, in the latter country, "Do you pound brass ? " is said to be a very common question. I have written the following pages in the hope that they might, in some measure, supply a want, and prove of service to my brother amateurs. It has been hinted to me that some of my chapters are rather "advanced;" in other words, that I have gone farther than amateurs are likely to follow me. -

Metals and Metal Products Tariff Schedules of the United States
251 SCHEDULE 6. - METALS AND METAL PRODUCTS TARIFF SCHEDULES OF THE UNITED STATES SCHEDULE 6. - METALS AND METAL PRODUCTS 252 Part 1 - Metal-Bearing Ores and Other Metal-Bearing Schedule 6 headnotes: Materials 1, This schedule does not cover — Part 2 Metals, Their Alloys, and Their Basic Shapes and Forms (II chemical elements (except thorium and uranium) and isotopes which are usefully radioactive (see A. Precious Metals part I3B of schedule 4); B. Iron or Steel (II) the alkali metals. I.e., cesium, lithium, potas C. Copper sium, rubidium, and sodium (see part 2A of sched D. Aluminum ule 4); or E. Nickel (lii) certain articles and parts thereof, of metal, F. Tin provided for in schedule 7 and elsewhere. G. Lead 2. For the purposes of the tariff schedules, unless the H. Zinc context requires otherwise — J. Beryllium, Columbium, Germanium, Hafnium, (a) the term "precious metal" embraces gold, silver, Indium, Magnesium, Molybdenum, Rhenium, platinum and other metals of the platinum group (iridium, Tantalum, Titanium, Tungsten, Uranium, osmium, palladium, rhodium, and ruthenium), and precious- and Zirconium metaI a Iloys; K, Other Base Metals (b) the term "base metal" embraces aluminum, antimony, arsenic, barium, beryllium, bismuth, boron, cadmium, calcium, chromium, cobalt, columbium, copper, gallium, germanium, Part 3 Metal Products hafnium, indium, iron, lead, magnesium, manganese, mercury, A. Metallic Containers molybdenum, nickel, rhenium, the rare-earth metals (Including B. Wire Cordage; Wire Screen, Netting and scandium and yttrium), selenium, silicon, strontium, tantalum, Fencing; Bale Ties tellurium, thallium, thorium, tin, titanium, tungsten, urani C. Metal Leaf and FoU; Metallics um, vanadium, zinc, and zirconium, and base-metal alloys; D, Nails, Screws, Bolts, and Other Fasteners; (c) the term "meta I" embraces precious metals, base Locks, Builders' Hardware; Furniture, metals, and their alloys; and Luggage, and Saddlery Hardware (d) in determining which of two or more equally specific provisions for articles "of iron or steel", "of copper", E. -

Piece Mold, Lost Wax & Composite Casting Techniques of The
Piece Mold, Lost Wax & Composite Casting Techniques of the Chinese Bronze Age Behzad Bavarian and Lisa Reiner Dept. of MSEM College of Engineering and Computer Science September 2006 Table of Contents Abstract Approximate timeline 1 Introduction 2 Bronze Transition from Clay 4 Elemental Analysis of Bronze Alloys 4 Melting Temperature 7 Casting Methods 8 Casting Molds 14 Casting Flaws 21 Lost Wax Method 25 Sanxingdui 28 Environmental Effects on Surface Appearance 32 Conclusion 35 References 36 China can claim a history rich in over 5,000 years of artistic, philosophical and political advancement. As well, it is birthplace to one of the world's oldest and most complex civilizations. By 1100 BC, a high level of artistic and technical skill in bronze casting had been achieved by the Chinese. Bronze artifacts initially were copies of clay objects, but soon evolved into shapes invoking bronze material characteristics. Essentially, the bronze alloys represented in the copper-tin-lead ternary diagram are not easily hot or cold worked and are difficult to shape by hammering, the most common techniques used by the ancient Europeans and Middle Easterners. This did not deter the Chinese, however, for they had demonstrated technical proficiency with hard, thin walled ceramics by the end of the Neolithic period and were able to use these skills to develop a most unusual casting method called the piece mold process. Advances in ceramic technology played an influential role in the progress of Chinese bronze casting where the piece mold process was more of a technological extension than a distinct innovation. Certainly, the long and specialized experience in handling clay was required to form the delicate inscriptions, to properly fit the molds together and to prevent them from cracking during the pour. -

The Lost-Wax Casting Process—Down to Basics by Eddie Bell, Founder, Santa Fe Symposium
The Lost-Wax Casting Process—Down To Basics By Eddie Bell, Founder, Santa Fe Symposium. Lost-wax casting is a ancient technique that is used today in essentially the same manner as it was first used more than 5,000 years ago. As they say, there's no messing with success. Today, of course, technology has vastly expanded the technique and produced powerful equipment that makes the process faster, easier and more productive than ever, but the basic steps remain the same. The steps below represent a simple overview and are intended to provide a beginning understanding of the casting process. Concept This is obviously where the design is initally conceived, discussed,evolved, and captured on paper—or on computer; CAD (computer aided design) software is increasingly popular among designers. You create the design you envision using the computer tool and the software creates a file that can be uploaded into a CNC mill or 3D printer. Model Build a model, either by hand-carving, guided by the paper rendering, or by uploading the CAD file into a computer controlled milling machine or a 3D printing machine. Models are made using carving wax, resin or similar material. This process can also be done in metal by a goldsmith or silversmith. Note: If a 3D printer or other rapid prototyping equipment is used, it is possible to skip the molding and wax-injection steps by using one of the resins that are specially made to go directly to the treeing process. Molding Create a mold from your master model, placing it in one of a variety of rubber or silicone materials, curing the material, then removing the model from the finished mold. -

Section XV BASE METALS and ARTICLES of BASE METAL Notes
Section XV BASE METALS AND ARTICLES OF BASE METAL Notes. 1.- This Section does not cover : (a) Prepared paints, inks or other products with a basis of metallic flakes or powder (headings 32.07 to 32.10, 32.12, 32.13 or 32.15); (b) Ferro-cerium or other pyrophoric alloys (heading 36.06); (c) Headgear or parts thereof of heading 65.06 or 65.07; (d) Umbrella frames or other articles of heading 66.03; (e) Goods of Chapter 71 (for example, precious metal alloys, base metal clad with precious metal, imitation jewellery); (f) Articles of Section XVI (machinery, mechanical appliances and electrical goods); (g) Assembled railway or tramway track (heading 86.08) or other articles of Section XVII (vehicles, ships and boats, aircraft); (h) Instruments or apparatus of Section XVIII, including clock or watch springs; (ij) Lead shot prepared for ammunition (heading 93.06) or other articles of Section XIX (arms and ammunition); (k) Articles of Chapter 94 (for example, furniture, mattress supports, lamps and lighting fittings, illuminated signs, prefabricated buildings); (l) Articles of Chapter 95 (for example, toys, games, sports requisites); (m) Hand sieves, buttons, pens, pencil-holders, pen nibs or other articles of Chapter 96 (miscellaneous manufactured articles); or (n) Articles of Chapter 97 (for example, works of art). 2.- Throughout the Nomenclature, the expression “parts of general use” means : (a) Articles of heading 73.07, 73.12, 73.15, 73.17 or 73.18 and similar articles of other base metal; (b) Springs and leaves for springs, of base metal, other than clock or watch springs (heading 91.14); and (c) Articles of headings 83.01, 83.02, 83.08, 83.10 and frames and mirrors, of base metal, of heading 83.06. -

Jay's Casting Demonstration
Making a Master to Cast & Process for Casting It October 6 2018: Jay Levy What is “casting”? Casting is a manufacturing process in which a liquid material is usually poured into a mold, which contains a hollow cavity of the desired shape, and then allowed to solidify. The solidified part is also known as a casting, which is ejected or broken out of the mold to complete the process. https://en.wikipedia.org/wiki/Casting Why cast? Process and concept – When creating jewelry, you consider casting if a piece is far too difficult to make with fabrication (metalsmithing) or when you want to produce many identical pieces using a reusable mold. Professional casters generally make castings faster and/or cheaper than you can. (fyi: Casters are lower paid than bench technicians or metalsmiths). How is a model for a casting created? Lost wax techniques are 5000-6000 years old with bee wax and clay used instead of wax. Apart from wax, your model can be anything that melts away clean. Rules for working with Wax: None or few. Don’t contaminate wax with things that don’t burn out – e.g., not bones; Flowers and pine cones are castable “doable” but the cast piece may be too heavy. Styrofoam gives off formaldehyde, only floral green foam (Sternofoam) doesn’t have formaldehyde. All metals shrink, typically 5-7%, except antimony which expands. Therefore, make models for metal rings ¼ size larger. On average, make other models about 10% larger. Edges are never clean on a casting. Consider rounding edges on outside and thin, carve out or fillet the inside. -

GLOSSARY of PLATING TERMS Acid Gold: a Mildly Acidic Process That Is Used When Plating from 7 to 200 Mils of Gold
GLOSSARY OF PLATING TERMS Acid gold: A mildly acidic process that is used when plating from 7 to 200 mils of gold. The deposits are usually 23kt purity, and it is not usually used as the final finish. Antique: A process which involves the application of a dark top coating over bronze or silver. This coating, either plated or painted, is partially removed to expose some of the underlying metal. Anti-tarnish: A protective coating that provides minimal tarnish protection for a low cost. Barrel plating: A type of mass finishing that takes place in a barrel or tub. Barrel plating is usually requested for very small pieces where pricing must be kept low. Black nickel: A bright or matte, dark plating process that is used to highlight antique finishes. Or, when used as a final color it will range from dark grey to light black. A bright black nickel will yield the darkest color. Bright finish: A high luster, smooth finish. CASS testing: The Copper-Accelerated Acetic Acid Salt Spray test is the same as the Neutral Salt Spray (NSS) test, except it is accelerated, with typical time cycles being 8 and 24 hours. Cold nickel: A non-brightened nickel bath which replicates the original finish, that is, bright areas remain bright and dull areas remain dull. Color: Describes the final top coating (flash) which could be white, silver, 14kt gold (Hamilton), 18kt gold, or 24kt gold (English gold). See "gold flash" and "cyanide gold." Copper: An excellent undercoat in the plating process. Copper provides good conductivity and forms an excellent protective barrier between the base metal and the plate. -

The Care and Preservation of Historical Silver by CLARA DECK, CONSERVATOR REVISIONS by LOUISE BECK, CONSERVATOR
The Care and Preservation of Historical Silver BY CLARA DECK, CONSERVATOR REVISIONS BY LOUISE BECK, CONSERVATOR Introduction Historical silver can be maintained for years of use and enjoyment provided that some basic care and attention is given to their preservation. The conservation staff at The Henry Ford have compiled the information in this fact sheet to help individuals care for their objects and collections. The first step in the care of all collections is to understand and minimize or eliminate conditions that can cause damage. The second step is to follow basic guidelines for care, handling and cleaning. Most people know that silver is a white, lustrous metal. Pure or “fine” silver is called “Sterling” if it is made up of no less than 925 parts silver to 75 parts alloy. Sterling will thus often have ‘.925’ stamped somewhere on it, as an identifier. Silver objects, especially coins and jewelry, contain copper as an alloying metal for added hardness. The copper may corrode to form dark brown or green deposits on the surface of the metal. Silver is usually easy to differentiate from lead or pewter, which are generally dark gray and not very shiny. Silver is often plated (deposited) onto other metallic alloys, almost always with an intermediate layer of copper in between. The earliest plating process, “Sheffield Plate” was developed in England in 1742. By the mid-19th century, the process was largely replaced by electroplating (which used less silver). The base metal in plated artifacts may consist of any of the following metals or alloys: copper, brass, “German silver” or “nickel silver” (50% copper, 30% nickel, 20% zinc), “Brittania metal” (97% tin, 7% antimony, 2% copper), or a “base” silver containing a high percentage of copper. -

A Decomposition Analysis of Base Metal Prices: Comparing the Effect of Detrending Methods on Trend Identification and Cyclical Components
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Dissertations, Master's Theses and Master's Reports - Open Reports 2013 A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS Mahmoodreza Modirroosta Michigan Technological University Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Economics Commons Copyright 2013 Mahmoodreza Modirroosta Recommended Citation Modirroosta, Mahmoodreza, "A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS", Master's Thesis, Michigan Technological University, 2013. https://doi.org/10.37099/mtu.dc.etds/674 Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Economics Commons A DECOMPOSITION ANALYSIS OF BASE METAL PRICES: COMPARING THE EFFECT OF DETRENDING METHODS ON TREND IDENTIFICATION AND CYCLICAL COMPONENTS By Mahmoodreza Modirroosta A THESIS Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Applied Natural Resource Economics MICHIGAN TECHNOLOGICAL UNIVERSITY 2013 © Mahmoodreza Modirroosta This thesis has been approved in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE in Applied Natural Resource Economics School of Business and Economics Thesis Advisor: Dr. Mark Roberts Committee Member: Dr. Xavier de Castro Oliveira Committee Member: Dr. Seyyedmohsen -

Casting Alloys: the Saga of Their Existence and the Recipe of Their Blend
Review Article International Journal of Dental Materials 2019; 1(1) Casting alloys: The saga of their existence and the recipe of their blend Guduri Vineeth1,*, Rama Krishna Alla2, Srinivasa Raju D3, Suresh Sajjan MC4, Ramaraju AV4, Harika Yeleti5, 1Senior Lecturer, Department of Prosthodontics, Vishnu Dental College, Bhimavaram, West Godavari, 534202, Andhra Pradesh, India. 2Assistant Professor, Department of Dental Materials, Vishnu Dental College, Bimavaram, West Godavari, 534202, Andhra Pradesh, India. 3Professor, Department of Dentistry, Maharaja Institute of Medical Sciences, Nellimarala, Vizainagaram,, Andhra Pradesh, India. 4Professor, Department of Prosthodontics, Vishnu Dental College, Bhimavaram, West Godavari, 534202, Andhra Pradesh, India. 5Senior Lecturer, Department of Prosthodontics, Lenora Institute of Dental Sciences, Rajahmundry, East Godavari District, Andhra Pradesh 533294, India. INFORMATIO N ABSTRACT Though a variety of metals and combinations have been in use since dec- Article History ades in the field of dentistry, there are only a few which have sustained the challenges of evolving material science. The history of these alloys, their Received 28 April 2019 constituent metals and properties impart the rationale of their use both in Received revised the past and advancing future perspectives. Also bearing the environmen- 11 May 2019 tal hazards in laboratory and clinical environments, safe levels of exposure Accepted 13 May 2019 to these alloys and aspects of selecting the best option among the different Available online 15 May 2019 alternatives is important. KEYWORDS 1. Introduction In dentistry, metals represent one of the four major classes of materials used Alloy for the reconstruction of damaged or missing oral tissues while the others Casting being ceramics, polymers and composites [1]. -
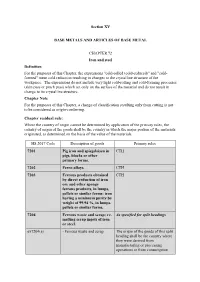
Section XV BASE METALS and ARTICLES of BASE METAL
Section XV BASE METALS AND ARTICLES OF BASE METAL CHAPTER 72 Iron and steel Definition For the purposes of this Chapter, the expressions "cold-rolled (cold-reduced)" and "cold- formed" mean cold reduction resulting in changes to the crystalline structure of the workpiece. The expressions do not include very light cold-rolling and cold-forming processes (skin pass or pinch pass) which act only on the surface of the material and do not result in change to its crystalline structure. Chapter Note For the purposes of this Chapter, a change of classification resulting only from cutting is not to be considered as origin-conferring. Chapter residual rule: Where the country of origin cannot be determined by application of the primary rules, the country of origin of the goods shall be the country in which the major portion of the materials originated, as determined on the basis of the value of the materials. HS 2017 Code Description of goods Primary rules 7201 Pig iron and spiegeleisen in CTH pigs, blocks or other primary forms. 7202 Ferro-alloys. CTH 7203 Ferrous products obtained CTH by direct reduction of iron ore and other spongy ferrous products, in lumps, pellets or similar forms; iron having a minimum purity by weight of 99.94 %, in lumps, pellets or similar forms. 7204 Ferrous waste and scrap; re- As specified for split headings melting scrap ingots of iron or steel. ex7204(a) - Ferrous waste and scrap The origin of the goods of this split heading shall be the country where they were derived from manufacturing or processing operations or from consumption HS 2017 Code Description of goods Primary rules ex7204(b) - Re-melting scrap ingots of The origin of the goods of this split iron or steel heading shall be the country where the waste and scrap used to obtain them were derived from manufacturing or processing operations or from consumption 7205 Granules and powders, of As specified for subheadings pig iron, spiegeleisen, iron or steel.