The Structure, Development and Role of Song in Dippers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Conservation Ecology of the European Nightjar (Caprimulgus Europaeus) in a Complex Heathland-Plantation Landscape
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of East Anglia digital repository The conservation ecology of the European nightjar (Caprimulgus europaeus) in a complex heathland-plantation landscape. Katrina Sharps A thesis submitted for the degree of Doctor of Philosophy at the School of Environmental Sciences, University of East Anglia, Norwich, UK. May 2013 © This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived there from must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. Acknowledgements Firstly, I would like to thank my primary supervisor Paul Dolman for his constant advice, support and enthusiasm throughout this PhD. I am also grateful to the other members of my supervisory team: Ian Henderson of the British Trust for Ornithology (BTO) and Andrew Lovett of UEA, for their useful guidance. Special thanks also go to Neal Armour-Chelu of the Forestry Commission and Greg Conway of the BTO for practical advice for the fieldwork and their invaluable experience and knowledge of forest management and working with nightjars respectively. Next, I would like to thank the other members of my radio-tracking and moth trapping teams – Vivien Hartwell, Laura Wilkinson, Elwyn Sharps, Alastair Feather, Kirsten Miller and Isobel Winney. Their efforts were tireless and they showed dedication to the project throughout. Additional thanks to all radio-tracking and nest finding volunteers, including Forestry Commission, RSPB and Wildlife Trust staff. -

Pre-Concept for a Regional Project ______
ADAPTATION FUND BOARD SECRETARIAT TECHNICAL REVIEW OF PROJECT/PROGRAMME PROPOSAL PROJECT/PROGRAMME CATEGORY: Pre-Concept for a Regional Project _________________________________________________________________________________________________________ Countries/Region: Costa Rica and Dominican Republic/ LAC Project Title: Improving the adaptive capacity of coastal communities in Costa Rica and the Dominican Republic through ecosystem-based adaptation strategies Thematic Focal Area: Ecosystem Based Adaptation (EbA) Implementing Entity: Development Bank of Latin America (CAF) Executing Entities: Sistema Nacional de Áreas de Conservación (Costa Rica) and Ministerio de Medio Ambiente y Recursos Naturales (Dominican Republic) AF Project ID: LAC/RIE/EBA/2020/PPC/1 IE Project ID: <IE to fill out> Requested Financing From Adaptation Fund (US Dollars): 13,919,202 Reviewer and contact person: Alyssa Gomes, Martina Dorigo (AFSEC) Co-reviewer(s): Jason Spensley (GEFSEC) IE Contact Person: <IE to fill out> Technical The project “Improving the adaptive capacity of coastal communities in Costa Rica and the Dominican Republic Summary through ecosystem-based adaptation strategies” aims to improve local adaptive capacity to reduce the vulnerability to climate change of Cocos and Catalina islands and the production sectors that depend on their ecosystem services. This will be done through the four components below: Project/Programme Background and Context: Component 1: Reduction oF main anthropogenic pressures (USD 6,590,000). Component 2: Conservation oF coral reefs (USD 525,000). Component 3: Insurance tools for emergency action (USD 3,550,000). Component 4: Knowledge management (USD 710,000). Requested Financing overview: Project/Programme Execution Cost: USD 1,513,150 Total Project/Programme Cost: USD 12,888,150 Implementing Fee: USD 1,031,052 Financing Requested: USD 13,919,202 The proposal does not include a request For a project Formulation grant. -

Top 40 Singles Top 40 Albums Closer Final Song Blonde Dangerous Woman 1 the Chainsmokers Feat
29 August 2016 CHART #2049 Top 40 Singles Top 40 Albums Closer Final Song Blonde Dangerous Woman 1 The Chainsmokers feat. Halsey 21 M0 1 Frank Ocean 21 Ariana Grande Last week 2 / 4 weeks Disruptor/SonyMusic Last week 24 / 6 weeks SonyMusic Last week - / 1 weeks BoysDon'tCry Last week 26 / 14 weeks Republic/Universal Cold Water Cheap Thrills Suicide Squad OST All Over The World: The Best Of 2 Major Lazer feat. Justin Bieber And... 22 Sia 2 Various 22 ELO Last week 1 / 5 weeks Gold / Because/Warner Last week 23 / 27 weeks Platinum x2 / Inertia/Rhythm Last week 1 / 3 weeks Atlantic/Warner Last week 24 / 19 weeks Gold / SonyMusic Let Me Love You This Girl 25 Lemonade 3 DJ Snake feat. Justin Bieber 23 Kungs And Cookin' On 3 Burners 3 Adele 23 Beyonce Last week 3 / 3 weeks Interscope/Universal Last week 22 / 8 weeks KungsMusic/Universal Last week 4 / 40 weeks Platinum x7 / XL/Rhythm Last week 34 / 18 weeks Gold / Columbia/SonyMusic Heathens Starving Views A Head Full Of Dreams 4 Twenty One Pilots 24 Hailee Steinfeld And Grey feat. Zedd 4 Drake 24 Coldplay Last week 4 / 10 weeks Gold / Atlantic/Warner Last week - / 1 weeks Republic/Universal Last week 3 / 17 weeks CashMoney/Universal Last week - / 33 weeks Gold / Parlophone/Warner Don't Worry Bout It Hurts So Good Blurryface Kaleidoscope World 5 Kings 25 Astrid S 5 Twenty One Pilots 25 The Chills Last week 7 / 7 weeks Gold / ArchAngel/Warner Last week 18 / 5 weeks Universal Last week 2 / 33 weeks Gold / FueledByRamen/Warner Last week - / 11 weeks FlyingNun/FlyingOut One Dance Never Be Like You Poi E: The Story Of Our Song Major Key 6 Drake feat. -

Karaoke Catalog Updated On: 11/01/2019 Sing Online on in English Karaoke Songs
Karaoke catalog Updated on: 11/01/2019 Sing online on www.karafun.com In English Karaoke Songs 'Til Tuesday What Can I Say After I Say I'm Sorry The Old Lamplighter Voices Carry When You're Smiling (The Whole World Smiles With Someday You'll Want Me To Want You (H?D) Planet Earth 1930s Standards That Old Black Magic (Woman Voice) Blackout Heartaches That Old Black Magic (Man Voice) Other Side Cheek to Cheek I Know Why (And So Do You) DUET 10 Years My Romance Aren't You Glad You're You Through The Iris It's Time To Say Aloha (I've Got A Gal In) Kalamazoo 10,000 Maniacs We Gather Together No Love No Nothin' Because The Night Kumbaya Personality 10CC The Last Time I Saw Paris Sunday, Monday Or Always Dreadlock Holiday All The Things You Are This Heart Of Mine I'm Not In Love Smoke Gets In Your Eyes Mister Meadowlark The Things We Do For Love Begin The Beguine 1950s Standards Rubber Bullets I Love A Parade Get Me To The Church On Time Life Is A Minestrone I Love A Parade (short version) Fly Me To The Moon 112 I'm Gonna Sit Right Down And Write Myself A Letter It's Beginning To Look A Lot Like Christmas Cupid Body And Soul Crawdad Song Peaches And Cream Man On The Flying Trapeze Christmas In Killarney 12 Gauge Pennies From Heaven That's Amore Dunkie Butt When My Ship Comes In My Own True Love (Tara's Theme) 12 Stones Yes Sir, That's My Baby Organ Grinder's Swing Far Away About A Quarter To Nine Lullaby Of Birdland Crash Did You Ever See A Dream Walking? Rags To Riches 1800s Standards I Thought About You Something's Gotta Give Home Sweet Home -

Top 40 Singles Top 40 Albums Closer Cool Girl 22, a Million Greatest Hits 1 the Chainsmokers Feat
10 October 2016 CHART #2055 Top 40 Singles Top 40 Albums Closer Cool Girl 22, A Million Greatest Hits 1 The Chainsmokers feat. Halsey 21 Tove Lo 1 Bon Iver 21 Guns N Roses Last week 1 / 10 weeks Platinum / Disruptor/SonyMusic Last week 18 / 8 weeks Universal Last week - / 1 weeks Jagjaguwar/Rhythm Last week - / 55 weeks Platinum x6 / Universal Starboy Don't Let Me Down Suicide Squad OST In The Lonely Hour: Drowning Shado... 2 The Weeknd feat. Daft Punk 22 The Chainsmokers feat. Daya 2 Various 22 Sam Smith Last week 5 / 2 weeks Republic/Universal Last week 22 / 30 weeks Platinum x2 / Disruptor/SonyMusi... Last week 3 / 9 weeks Atlantic/Warner Last week 19 / 124 weeks Platinum x4 / Capitol/Universal Side To Side This Is What You Came For Purpose A Seat At The Table 3 Ariana Grande feat. Nicki Minaj 23 Calvin Harris feat. Rihanna 3 Justin Bieber 23 Solange Last week 2 / 5 weeks Gold / Republic/Universal Last week 21 / 23 weeks Platinum / Columbia/SonyMusic Last week 8 / 47 weeks Platinum / DefJam/Universal Last week - / 1 weeks Columbia/SonyMusic Cold Water This Town Westway (The Glitter And The Slums... Bridget Jones's Baby OST 4 Major Lazer feat. Justin Bieber And... 24 Niall Horan 4 Sticky Fingers 24 Various Last week 3 / 11 weeks Platinum / MadDecent/Warner Last week - / 1 weeks Capitol/Universal Last week - / 1 weeks Sureshaker/Warner Last week 17 / 3 weeks Polydor/Universal Let Me Love You Too Good Keep Me Singing Dangerous Woman 5 DJ Snake feat. Justin Bieber 25 Drake feat. -

Top 40 Singles Top 40 Albums Rockstar Thunder Beautiful Trauma Colors 1 Post Malone Feat
30 October 2017 CHART #2110 Top 40 Singles Top 40 Albums Rockstar Thunder Beautiful Trauma Colors 1 Post Malone feat. 21 Savage 21 Imagine Dragons 1 Pink 21 Beck Last week 1 / 6 weeks Gold / Republic/Universal Last week 20 / 23 weeks Platinum / Interscope/Universal Last week 1 / 2 weeks Gold / RCA/SonyMusic Last week 8 / 2 weeks Fonograf/Capitol/Universal Havana Homemade Dynamite (Remix) Divide 24K Magic 2 Camila Cabello feat. Young Thug 22 Lorde feat. Khalid, Post Malone And... 2 Ed Sheeran 22 Bruno Mars Last week 2 / 9 weeks Gold / Simco/SonyMusic Last week 21 / 6 weeks Universal Last week 2 / 34 weeks Platinum x5 / Asylum/Warner Last week 20 / 49 weeks Platinum / Atlantic/Warner Young, Dumb And Broke Friends Flicker Starboy 3 Khalid 23 Justin Bieber And BloodPop 3 Niall Horan 23 The Weeknd Last week 3 / 11 weeks Platinum / RCA/SonyMusic Last week 23 / 10 weeks Gold / Republic/Universal Last week - / 1 weeks NeonHazeMusic/Universal Last week 26 / 48 weeks Republic/Universal New Rules Bodak Yellow Stoney Purpose 4 Dua Lipa 24 Cardi B 4 Post Malone 24 Justin Bieber Last week 4 / 13 weeks Platinum / WEA/Warner Last week 24 / 6 weeks KSR/Atlantic/Warner Last week 3 / 43 weeks Republic/Universal Last week 25 / 102 weeks Platinum x2 / DefJam/Universal Silence Shape Of You Listen Without Prejudice: MTV Unplu... More Life 5 Marshmello feat. Khalid 25 Ed Sheeran 5 George Michael 25 Drake Last week 6 / 9 weeks Gold / RCA/SonyMusic Last week 25 / 42 weeks Platinum x4 / Asylum/Warner Last week - / 26 weeks SonyMusic Last week 27 / 32 weeks CashMoney/Universal What Lovers Do Jocelyn Flores Greatest Hits Now 6 Maroon 5 feat. -

First Documentation of Combinatorial Song Syntax in a Suboscine Passerine Species
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications, Department of Psychology Psychology, Department of 2005 FIRST DOCUMENTATION OF COMBINATORIAL SONG SYNTAX IN A SUBOSCINE PASSERINE SPECIES Daniel Leger University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/psychfacpub Part of the Psychiatry and Psychology Commons Leger, Daniel, "FIRST DOCUMENTATION OF COMBINATORIAL SONG SYNTAX IN A SUBOSCINE PASSERINE SPECIES" (2005). Faculty Publications, Department of Psychology. 476. https://digitalcommons.unl.edu/psychfacpub/476 This Article is brought to you for free and open access by the Psychology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications, Department of Psychology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The Condor 107:765±774 q The Cooper Ornithological Society 2005 FIRST DOCUMENTATION OF COMBINATORIAL SONG SYNTAX IN A SUBOSCINE PASSERINE SPECIES DANIEL W. L EGER1 Department of Psychology and Nebraska Behavioral Biology Group, University of Nebraska, Lincoln, NE 68588-0308 Abstract. Birds with songs having two or more acoustically distinct elements can arrange them either rigidly (i.e., in the same sequence) or ¯exibly. Flexible song syntax can be achieved either by varying the number of repetitions of elements or by combining elements in different ways. Combinatorial syntax has been documented only in the songs of oscine passerines and in one nonpasserine, but not in the suboscine passerines. Dawn and day songs of a tyrant ¯ycatcher, the Flammulated Attila (Attila ¯ammulatus), were recorded in Costa Rica. Flexible syntax was noted in both dawn and day song. -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -

Tai Poutini Polytechnic Media Release Joel Little 21 October 2014
Tai Poutini Polytechnic Media Release Joel Little 21 October 2014 Former MAINZ student Joel Little has capped-off an extraordinary year with top New Zealand accolades for his work on Lorde’s Pure Heroine album. Joel has won Best Producer and Best Engineer in the technical categories of the Vodafone NZ Music Awards. He was among a number of former MAINZ students to be recognised at the event last week, which revealed winners in the technical categories and the finalists for the Tuis in November. Joel’s career started with the Diploma in Contemporary Music Performance at MAINZ. He was lead singer and guitarist with Goodnight Nurse, but as his career evolved, focused more on songwriting, producing and nurturing new talent, including up-and-coming pop duo Broods, also recognised at last week’s awards. The past year has been a big one for Joel, as his collaboration with Lorde has seen him step into the global spotlight. Lorde’s hit single Royals, co-written and produced by Joel, surged to the top of the charts, won them the 2013 APRA Silver Scroll Award for songwriting excellence and then scooped the 2014 Grammy for Song of the Year. Success means embracing a more international lifestyle, but even with projects on the go in London and LA, he still considers Auckland home. Joel has maintained a connection with MAINZ, saying: ‘I loved being able to make music all day, it was the only thing I wanted to do so having all the facilities at MAINZ was great. I appreciate the leg up that MAINZ gave me in terms of having that basic understanding of the business and the industry. -

Holocene Vertebrate Fossils from Isla Floreana, Galapagos
Holocene Vertebrate Fossils from Isla Floreana, Galapagos DAVID W. STEADMAN SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 413 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

American Assassin By Vince Flynn (Suspense) Syracuse University
Grade 12 - Summer Choices Program 2020 (NOT including AP Courses) Incoming seniors (not enrolled in an AP English course) must select ONE of the following texts to read over the summer. Please select books which you have not previously read. Assessments will be administered in the fall for the text selected. Students enrolled in AP courses should refer to their respective course lists. American Assassin by Vince Flynn (Suspense) Syracuse University student Mitch Rapp broods over the deaths of his girlfriend and thirty-four other classmates on the bombed Pan Am Flight 103, until he is recruited and trained by the CIA and receives his first assignment--to kill the Turkish arms dealer who sold the bomb that killed his friends. Between the World and Me by Ta-Nehisi Coates (Autobiographical) At every stage of Ta-Nehisi Coates’ life, he's sought in his explorations of history answers to the mysteries that surrounded him--most urgently, why he, and other black people he knew, seemed to live in fear. Coates takes readers along on his journey through America's history of race and its contemporary resonances through a series of awakenings. Beyond Belief: Finding the Strength to Come Back by Josh Hamilton with Tim Keown (Autobiography) Josh Hamilton was the first player chosen in the first round of the 1999 baseball draft. He was destined to be one of those rare "high-character " superstars. But in 2001, working his way from the minors to the majors, all of the plans for Josh went off the rails in a moment of weakness. -
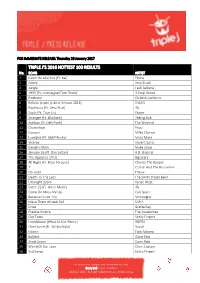
Triple J's 2016 Hottest 100 Results
FOR IMMEDIATE RELEASE: Thursday 26 January 2017 TRIPLE J’S 2016 HOTTEST 100 RESULTS No. SONG ARTIST 1 Never Be Like You {Ft. Kai} Flume 2 Adore Amy Shark 3 Jungle Tash Sultana 4 1955 {Ft. Montaigne/Tom Thum} Hilltop Hoods 5 Redbone Childish Gambino 6 Believe {triple j Like A Version 2016} DMA'S 7 Papercuts {Ft. Vera Blue} Illy 8 Say It {Ft. Tove Lo} Flume 9 Stranger {Ft. Elliphant} Peking Duk 10 Starboy {Ft. Daft Punk} The Weeknd 11 Chameleon Pnau 12 Cocoon Milky Chance 13 Love$ick {Ft. A$AP Rocky} Mura Masa 14 Viceroy Violent Soho 15 Genghis Khan Miike Snow 16 January 26 {Ft. Dan Sultan} A.B. Original 17 The Opposite Of Us Big Scary 18 All Night {Ft. Knox Fortune} Chance The Rapper 19 7 Catfish And The Bottlemen 20 On Hold The xx 21 Death To The Lads The Smith Street Band 22 Ultralight Beam Kanye West 23 Catch 22 {Ft. Anne-Marie} Illy 24 Come On Mess Me Up Cub Sport 25 Because I Love You Montaigne 26 Make Them Wheels Roll SAFIA 27 Drive Gretta Ray 28 Frankie Sinatra The Avalanches 29 Our Town Sticky Fingers 30 Innerbloom {What So Not Remix} RÜFÜS 31 One Dance {Ft. Wizkid/Kyla} Drake 32 Notion Tash Sultana 33 Bullshit Dune Rats 34 Scott Green Dune Rats 35 World Of Our Love Client Liaison 36 Sad Songs Sticky Fingers For more info, images and interviews contact: Gerry Bull, triple j Publicist 02 8333 1641 | [email protected] | triplej.net.au 37 Smoke & Retribution {Ft.