Second Broods in Bobwhite Quail
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Structure, Development and Role of Song in Dippers
The structure, development and role of song in dippers. Lucy Magoolagan Thesis submitted for the degree of Doctor of Philosophy April 2017 This thesis is my own work, and has not been submitted in substantially the same form for the award of a higher degree elsewhere. Word count: 41,886 Contents Acknowledgements Chapter 1: General introduction ....................................................................................................1 1.1 Introduction ..........................................................................................................................1 1.1.1 Male song .....................................................................................................................2 1.1.2 Female song ..................................................................................................................3 1.2 Song development ................................................................................................................4 1.3 The developmental stress hypothesis ..................................................................................6 1.3.1 Brood size ....................................................................................................................7 1.3.2 Parental care .................................................................................................................7 1.3.3 Weather .........................................................................................................................8 1.3.4 Parasite load ..................................................................................................................8 -

Top 40 Singles Top 40 Albums Closer Final Song Blonde Dangerous Woman 1 the Chainsmokers Feat
29 August 2016 CHART #2049 Top 40 Singles Top 40 Albums Closer Final Song Blonde Dangerous Woman 1 The Chainsmokers feat. Halsey 21 M0 1 Frank Ocean 21 Ariana Grande Last week 2 / 4 weeks Disruptor/SonyMusic Last week 24 / 6 weeks SonyMusic Last week - / 1 weeks BoysDon'tCry Last week 26 / 14 weeks Republic/Universal Cold Water Cheap Thrills Suicide Squad OST All Over The World: The Best Of 2 Major Lazer feat. Justin Bieber And... 22 Sia 2 Various 22 ELO Last week 1 / 5 weeks Gold / Because/Warner Last week 23 / 27 weeks Platinum x2 / Inertia/Rhythm Last week 1 / 3 weeks Atlantic/Warner Last week 24 / 19 weeks Gold / SonyMusic Let Me Love You This Girl 25 Lemonade 3 DJ Snake feat. Justin Bieber 23 Kungs And Cookin' On 3 Burners 3 Adele 23 Beyonce Last week 3 / 3 weeks Interscope/Universal Last week 22 / 8 weeks KungsMusic/Universal Last week 4 / 40 weeks Platinum x7 / XL/Rhythm Last week 34 / 18 weeks Gold / Columbia/SonyMusic Heathens Starving Views A Head Full Of Dreams 4 Twenty One Pilots 24 Hailee Steinfeld And Grey feat. Zedd 4 Drake 24 Coldplay Last week 4 / 10 weeks Gold / Atlantic/Warner Last week - / 1 weeks Republic/Universal Last week 3 / 17 weeks CashMoney/Universal Last week - / 33 weeks Gold / Parlophone/Warner Don't Worry Bout It Hurts So Good Blurryface Kaleidoscope World 5 Kings 25 Astrid S 5 Twenty One Pilots 25 The Chills Last week 7 / 7 weeks Gold / ArchAngel/Warner Last week 18 / 5 weeks Universal Last week 2 / 33 weeks Gold / FueledByRamen/Warner Last week - / 11 weeks FlyingNun/FlyingOut One Dance Never Be Like You Poi E: The Story Of Our Song Major Key 6 Drake feat. -

Karaoke Catalog Updated On: 11/01/2019 Sing Online on in English Karaoke Songs
Karaoke catalog Updated on: 11/01/2019 Sing online on www.karafun.com In English Karaoke Songs 'Til Tuesday What Can I Say After I Say I'm Sorry The Old Lamplighter Voices Carry When You're Smiling (The Whole World Smiles With Someday You'll Want Me To Want You (H?D) Planet Earth 1930s Standards That Old Black Magic (Woman Voice) Blackout Heartaches That Old Black Magic (Man Voice) Other Side Cheek to Cheek I Know Why (And So Do You) DUET 10 Years My Romance Aren't You Glad You're You Through The Iris It's Time To Say Aloha (I've Got A Gal In) Kalamazoo 10,000 Maniacs We Gather Together No Love No Nothin' Because The Night Kumbaya Personality 10CC The Last Time I Saw Paris Sunday, Monday Or Always Dreadlock Holiday All The Things You Are This Heart Of Mine I'm Not In Love Smoke Gets In Your Eyes Mister Meadowlark The Things We Do For Love Begin The Beguine 1950s Standards Rubber Bullets I Love A Parade Get Me To The Church On Time Life Is A Minestrone I Love A Parade (short version) Fly Me To The Moon 112 I'm Gonna Sit Right Down And Write Myself A Letter It's Beginning To Look A Lot Like Christmas Cupid Body And Soul Crawdad Song Peaches And Cream Man On The Flying Trapeze Christmas In Killarney 12 Gauge Pennies From Heaven That's Amore Dunkie Butt When My Ship Comes In My Own True Love (Tara's Theme) 12 Stones Yes Sir, That's My Baby Organ Grinder's Swing Far Away About A Quarter To Nine Lullaby Of Birdland Crash Did You Ever See A Dream Walking? Rags To Riches 1800s Standards I Thought About You Something's Gotta Give Home Sweet Home -

Top 40 Singles Top 40 Albums Closer Cool Girl 22, a Million Greatest Hits 1 the Chainsmokers Feat
10 October 2016 CHART #2055 Top 40 Singles Top 40 Albums Closer Cool Girl 22, A Million Greatest Hits 1 The Chainsmokers feat. Halsey 21 Tove Lo 1 Bon Iver 21 Guns N Roses Last week 1 / 10 weeks Platinum / Disruptor/SonyMusic Last week 18 / 8 weeks Universal Last week - / 1 weeks Jagjaguwar/Rhythm Last week - / 55 weeks Platinum x6 / Universal Starboy Don't Let Me Down Suicide Squad OST In The Lonely Hour: Drowning Shado... 2 The Weeknd feat. Daft Punk 22 The Chainsmokers feat. Daya 2 Various 22 Sam Smith Last week 5 / 2 weeks Republic/Universal Last week 22 / 30 weeks Platinum x2 / Disruptor/SonyMusi... Last week 3 / 9 weeks Atlantic/Warner Last week 19 / 124 weeks Platinum x4 / Capitol/Universal Side To Side This Is What You Came For Purpose A Seat At The Table 3 Ariana Grande feat. Nicki Minaj 23 Calvin Harris feat. Rihanna 3 Justin Bieber 23 Solange Last week 2 / 5 weeks Gold / Republic/Universal Last week 21 / 23 weeks Platinum / Columbia/SonyMusic Last week 8 / 47 weeks Platinum / DefJam/Universal Last week - / 1 weeks Columbia/SonyMusic Cold Water This Town Westway (The Glitter And The Slums... Bridget Jones's Baby OST 4 Major Lazer feat. Justin Bieber And... 24 Niall Horan 4 Sticky Fingers 24 Various Last week 3 / 11 weeks Platinum / MadDecent/Warner Last week - / 1 weeks Capitol/Universal Last week - / 1 weeks Sureshaker/Warner Last week 17 / 3 weeks Polydor/Universal Let Me Love You Too Good Keep Me Singing Dangerous Woman 5 DJ Snake feat. Justin Bieber 25 Drake feat. -

Top 40 Singles Top 40 Albums Rockstar Thunder Beautiful Trauma Colors 1 Post Malone Feat
30 October 2017 CHART #2110 Top 40 Singles Top 40 Albums Rockstar Thunder Beautiful Trauma Colors 1 Post Malone feat. 21 Savage 21 Imagine Dragons 1 Pink 21 Beck Last week 1 / 6 weeks Gold / Republic/Universal Last week 20 / 23 weeks Platinum / Interscope/Universal Last week 1 / 2 weeks Gold / RCA/SonyMusic Last week 8 / 2 weeks Fonograf/Capitol/Universal Havana Homemade Dynamite (Remix) Divide 24K Magic 2 Camila Cabello feat. Young Thug 22 Lorde feat. Khalid, Post Malone And... 2 Ed Sheeran 22 Bruno Mars Last week 2 / 9 weeks Gold / Simco/SonyMusic Last week 21 / 6 weeks Universal Last week 2 / 34 weeks Platinum x5 / Asylum/Warner Last week 20 / 49 weeks Platinum / Atlantic/Warner Young, Dumb And Broke Friends Flicker Starboy 3 Khalid 23 Justin Bieber And BloodPop 3 Niall Horan 23 The Weeknd Last week 3 / 11 weeks Platinum / RCA/SonyMusic Last week 23 / 10 weeks Gold / Republic/Universal Last week - / 1 weeks NeonHazeMusic/Universal Last week 26 / 48 weeks Republic/Universal New Rules Bodak Yellow Stoney Purpose 4 Dua Lipa 24 Cardi B 4 Post Malone 24 Justin Bieber Last week 4 / 13 weeks Platinum / WEA/Warner Last week 24 / 6 weeks KSR/Atlantic/Warner Last week 3 / 43 weeks Republic/Universal Last week 25 / 102 weeks Platinum x2 / DefJam/Universal Silence Shape Of You Listen Without Prejudice: MTV Unplu... More Life 5 Marshmello feat. Khalid 25 Ed Sheeran 5 George Michael 25 Drake Last week 6 / 9 weeks Gold / RCA/SonyMusic Last week 25 / 42 weeks Platinum x4 / Asylum/Warner Last week - / 26 weeks SonyMusic Last week 27 / 32 weeks CashMoney/Universal What Lovers Do Jocelyn Flores Greatest Hits Now 6 Maroon 5 feat. -

Tai Poutini Polytechnic Media Release Joel Little 21 October 2014
Tai Poutini Polytechnic Media Release Joel Little 21 October 2014 Former MAINZ student Joel Little has capped-off an extraordinary year with top New Zealand accolades for his work on Lorde’s Pure Heroine album. Joel has won Best Producer and Best Engineer in the technical categories of the Vodafone NZ Music Awards. He was among a number of former MAINZ students to be recognised at the event last week, which revealed winners in the technical categories and the finalists for the Tuis in November. Joel’s career started with the Diploma in Contemporary Music Performance at MAINZ. He was lead singer and guitarist with Goodnight Nurse, but as his career evolved, focused more on songwriting, producing and nurturing new talent, including up-and-coming pop duo Broods, also recognised at last week’s awards. The past year has been a big one for Joel, as his collaboration with Lorde has seen him step into the global spotlight. Lorde’s hit single Royals, co-written and produced by Joel, surged to the top of the charts, won them the 2013 APRA Silver Scroll Award for songwriting excellence and then scooped the 2014 Grammy for Song of the Year. Success means embracing a more international lifestyle, but even with projects on the go in London and LA, he still considers Auckland home. Joel has maintained a connection with MAINZ, saying: ‘I loved being able to make music all day, it was the only thing I wanted to do so having all the facilities at MAINZ was great. I appreciate the leg up that MAINZ gave me in terms of having that basic understanding of the business and the industry. -

American Assassin By Vince Flynn (Suspense) Syracuse University
Grade 12 - Summer Choices Program 2020 (NOT including AP Courses) Incoming seniors (not enrolled in an AP English course) must select ONE of the following texts to read over the summer. Please select books which you have not previously read. Assessments will be administered in the fall for the text selected. Students enrolled in AP courses should refer to their respective course lists. American Assassin by Vince Flynn (Suspense) Syracuse University student Mitch Rapp broods over the deaths of his girlfriend and thirty-four other classmates on the bombed Pan Am Flight 103, until he is recruited and trained by the CIA and receives his first assignment--to kill the Turkish arms dealer who sold the bomb that killed his friends. Between the World and Me by Ta-Nehisi Coates (Autobiographical) At every stage of Ta-Nehisi Coates’ life, he's sought in his explorations of history answers to the mysteries that surrounded him--most urgently, why he, and other black people he knew, seemed to live in fear. Coates takes readers along on his journey through America's history of race and its contemporary resonances through a series of awakenings. Beyond Belief: Finding the Strength to Come Back by Josh Hamilton with Tim Keown (Autobiography) Josh Hamilton was the first player chosen in the first round of the 1999 baseball draft. He was destined to be one of those rare "high-character " superstars. But in 2001, working his way from the minors to the majors, all of the plans for Josh went off the rails in a moment of weakness. -
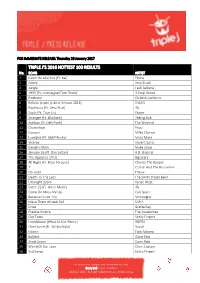
Triple J's 2016 Hottest 100 Results
FOR IMMEDIATE RELEASE: Thursday 26 January 2017 TRIPLE J’S 2016 HOTTEST 100 RESULTS No. SONG ARTIST 1 Never Be Like You {Ft. Kai} Flume 2 Adore Amy Shark 3 Jungle Tash Sultana 4 1955 {Ft. Montaigne/Tom Thum} Hilltop Hoods 5 Redbone Childish Gambino 6 Believe {triple j Like A Version 2016} DMA'S 7 Papercuts {Ft. Vera Blue} Illy 8 Say It {Ft. Tove Lo} Flume 9 Stranger {Ft. Elliphant} Peking Duk 10 Starboy {Ft. Daft Punk} The Weeknd 11 Chameleon Pnau 12 Cocoon Milky Chance 13 Love$ick {Ft. A$AP Rocky} Mura Masa 14 Viceroy Violent Soho 15 Genghis Khan Miike Snow 16 January 26 {Ft. Dan Sultan} A.B. Original 17 The Opposite Of Us Big Scary 18 All Night {Ft. Knox Fortune} Chance The Rapper 19 7 Catfish And The Bottlemen 20 On Hold The xx 21 Death To The Lads The Smith Street Band 22 Ultralight Beam Kanye West 23 Catch 22 {Ft. Anne-Marie} Illy 24 Come On Mess Me Up Cub Sport 25 Because I Love You Montaigne 26 Make Them Wheels Roll SAFIA 27 Drive Gretta Ray 28 Frankie Sinatra The Avalanches 29 Our Town Sticky Fingers 30 Innerbloom {What So Not Remix} RÜFÜS 31 One Dance {Ft. Wizkid/Kyla} Drake 32 Notion Tash Sultana 33 Bullshit Dune Rats 34 Scott Green Dune Rats 35 World Of Our Love Client Liaison 36 Sad Songs Sticky Fingers For more info, images and interviews contact: Gerry Bull, triple j Publicist 02 8333 1641 | [email protected] | triplej.net.au 37 Smoke & Retribution {Ft. -

Pdf Download 2.9 MB
1. Lorde’s single “Royals” is the most successful single by a New Zealand artist of all time! But, her latest single is from a movie. What is the movie? BONUS: What other songs from movies have you heard? Why do you think it’s important for movies have songs and music in them? SONG: Lorde, “Yellow Flicker Beat” 2. These five Kiwi groups have something in common – they’re all family! Match the group to the right family members: Sol3 Mio Stan Walker and Ria Hall Broods Kora Adeaze Brother and Sister Brothers Brothers and Cousin Father and sons Cousin BONUS: Which members of your family play musical instruments or sing? What do they like to play and where do they do it? SONG: “Strong on My Own” – Adeaze Featuring Aaradhna 3. Four musicians came together in in 2014 to write a song in Te Reo Maori called “Aotearoa.” Maisey Rika, Ria Hall, Troy Kingi and Stan Walker. (left to right) Match the word in Te Reo with the word in English: 1. whenua a. ocean 2. tātou b. love 3. moana c. land 4. tūpuna d. together 5. aroha e. ancestor BONUS: This song is about being proud of Aotearoa/ New Zealand and our connection to the land. What makes you proud and happy to live in New Zealand? SONG: “Aotearoa” by Maisey Rika, Ria Hall, Troy Kingi and Stan Walker. 4. Benny Tipene became famous from which TV competition show? BONUS: If you had the chance to sing in a TV competition, what song would you sing and why? SONG: “Walking on Water” by Benny Tipene 5. -

Outcomes & Impacts 2011-2015
OUTCOMES & IMPACTS 2011-2015 October 2015 PURPOSE This is a report on the results of our Making Tracks music funding scheme over the four years since the scheme was established. The report is in two parts. Part A reports on the broadcast “mileage” achieved by Making Tracks-funded songs, on air and online, over the last four years. Part B reports on the artists we have funded and the impact on the airplay charts of Making Tracks-funded songs and artists. PART A: SPINS & STREAMS INTRODUCTION 1. The Making Tracks music funding scheme is now four years old. Every year, we do a count of the number of times Making Tracks-funded songs have played, both on air and online, giving us a raw measure of broadcast “mileage” achieved via the Making Tracks scheme. 2. This report gives a gauge of audience reached and from a financial point of view, value for money. We use it to review trends and spot strengths and weaknesses. 3. We have now completed our count for the first four years of the scheme. The count includes not only songs funded and released in the last financial year but also updates the spins and stream counts for songs funded in 2011-2012, 2012-2013 and 2013-2014. TOPLINE RESULTS 4. In the four years to 30 June 2015, we have funded 980 songs (after writebacks). So far, 831 or 85% of those songs have been released (with the rest still in production at time of writing). 5. Those 831 songs have amassed 109,557,019 spins on radio or music television, and streams online on measured platforms over the last four years. -

Gig” “This Won’T TICKETS Be Just MONKEYSARCTICTO SEE
Gruff Rhys Pete Doherty Wolf Alice SECRET ALBUM DETAILS! Tune-Yards “I’ve been told not to tell but…” 24 MAY 2014 MAY 24 The Streets IAN BROWN IAN “This won’t Arctic be just another gig” Monkeys “It’s not where you’re from, it’s where you’re at...” on their biggest weekend yet and what lies beyond ***R U going to be down the front?*** Interviews with the stellar Finsbury Park line-up Miles Kane Tame Impala Royal Blood The Amazing Snakeheads TICKETS TO SEE On the town with Arctics’ THE PAST, PRESENT favourite new band ARCTIC & FUTURE OF MUSIC WIN 24 MAY 2014 | £2.50 HEADLINE US$8.50 | ES€3.90 | CN$6.99 MONKEYS BLACK YELLOW MAGENTA CYAN 93NME14021105.pgs 16.05.2014 12:29 BLACK YELLOW MAGENTA CYAN 93NME14018164.pgs 25.04.2014 13:55 ESSENTIAL TRACKS NEW MUSICAL EXPRESS | 24 MAY 2014 4 SOUNDING OFF 8 THE WEEK THIS WEEK 16 IN THE STUDIO Pulled Apart By Horses 17 ANATOMY OF AN ALBUM The Streets – WE ASK… ‘A Grand Don’t Come For Free’ NEW 19 SOUNDTRACK OF MY LIFE Anton Newcombe, The Brian Jonestown Massacre BANDS TO DISCOVER 24 REVIEWS 40 NME GUIDE → THE BAND LIST 65 THIS WEEK IN… Alice Cooper 29 Lykke Li 6, 37 Anna Calvi 7 Lyves 21 66 THINK TANK The Amazing Mac Miller 6 Snakeheads 52 Manic Street Arctic Monkeys 44 Preachers 7 HOW’s PETE SPENDING Art Trip And The Michael Jackson 28 ▼FEATURES Static Sound 21 Miles Kane 50 Benjamin Booker 20 Mona & Maria 21 HIS TIME BEFORE THE Blaenavon 32 Morrissey 6 Bo Ningen 33 Mythhs 22 The Brian Jonestown Nick Cave 7 Massacre 19 Nihilismus 21 LIBS’ COMEBACK? Chance The Rapper 7 Oliver Wilde 37 Charli XCX 32 Owen Pallett 27 He’s putting together a solo Chelsea Wolfe 21 Palm Honey 22 8 Cheerleader 7 Peace 32 record in Hamburg Clap Your Hands Phoria 22 Say Yeah 7 Poliça 6 The Coral 15 Pulled Apart By 3 Courtly Love 22 Horses 16 IS THERE FINALLY Courtney Barnett 31 Ratking 33 Arctic Monkeys Courtney Love 36 Rhodes 6 Barry Nicolson gets the skinny Cymbals Eat Guitars 7 Royal Blood 51 A GOOD Darlia 40 Röyksopp & Robyn 27 on their upcoming Finsbury Echo & The San Mei 22 Park shows. -

NZ Music Month Song of the Day Fact Sheet Use with Our NZ Music Month Song of the Day Calendar and Playlist on Spotify and Youtube!
NZ Music Month Song of the Day Fact Sheet Use with our NZ Music Month Song of the Day calendar and playlist on Spotify and YouTube! Get ready for NZ Music Month! Learn the 2015 Hook, Line and Sing-along Song “Stickytarday” by Bethlehem College. Download it at www.hooklineandsingalong.com D Pluck from Bethlehem College, “Stickytarday” D Pluck is a five-piece band consisting of Josh Dodanduwa, Jethro Wall, Samuel Tanner, Mackenzie Lines and Daniel Cossey (Year 10) from Tauranga’s Bethlehem College. The boys won the 2015 Hook, Line and Sing-along songwriting competition with their song “Stickytarday”. Recorded and produced by Ben King at Auckland’s prestigious Roundhead Studios, the song will be sung at schools May 29 all over the nation, as part of NZ Music Month. 1. Dragon, “April Sun in Cuba” Dragon was a rock band in the 1970s and were inducted into the NZ Music Hall of Fame in 2011. “April Sun in Cuba” was a hit both in New Zealand and Australia, reaching #9 and #2 on the charts respectively. 2. Prince Tui Teka, "E Ipo" Prince Tui Teka, born in the heart of Te Urewera, wrote this song with the help of Ngoi Pēwhairangi, a Maori educator, teacher, translator who also wrote the lyrics to “Poi E” for The Patea Maori Club. This was the first song in Te Reo to reach #1 on the charts! The song was based on a popular Indonesian melody that Teka had listened to while performing overseas. 3. Dave Dobbyn, "Slice of Heaven" “Slice of Heaven” was written for the movie Footrot Flats: The Dog’s Tale, which was based on the iconic New Zealand cartoon series.