Species Assessment for Sage Thrasher (Oreoscoptes Montanus) in Wyoming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sonoran Joint Venture Bird Conservation Plan Version 1.0
Sonoran Joint Venture Bird Conservation Plan Version 1.0 Sonoran Joint Venture 738 N. 5th Avenue, Suite 102 Tucson, AZ 85705 520-882-0047 (phone) 520-882-0037 (fax) www.sonoranjv.org May 2006 Sonoran Joint Venture Bird Conservation Plan Version 1.0 ____________________________________________________________________________________________ Acknowledgments We would like to thank all of the members of the Sonoran Joint Venture Technical Committee for their steadfast work at meetings and for reviews of this document. The following Technical Committee meetings were devoted in part or total to working on the Bird Conservation Plan: Tucson, June 11-12, 2004; Guaymas, October 19-20, 2004; Tucson, January 26-27, 2005; El Palmito, June 2-3, 2005, and Tucson, October 27-29, 2005. Another major contribution to the planning process was the completion of the first round of the northwest Mexico Species Assessment Process on May 10-14, 2004. Without the data contributed and generated by those participants we would not have been able to successfully assess and prioritize all bird species in the SJV area. Writing the Conservation Plan was truly a group effort of many people representing a variety of agencies, NGOs, and universities. Primary contributors are recognized at the beginning of each regional chapter in which they participated. The following agencies and organizations were involved in the plan: Arizona Game and Fish Department, Audubon Arizona, Centro de Investigación Cientifica y de Educación Superior de Ensenada (CICESE), Centro de Investigación de Alimentación y Desarrollo (CIAD), Comisión Nacional de Áreas Naturales Protegidas (CONANP), Instituto del Medio Ambiente y el Desarrollo (IMADES), PRBO Conservation Science, Pronatura Noroeste, Proyecto Corredor Colibrí, Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Sonoran Institute, The Hummingbird Monitoring Network, Tucson Audubon Society, U.S. -

The Relationships of the Starlings (Sturnidae: Sturnini) and the Mockingbirds (Sturnidae: Mimini)
THE RELATIONSHIPS OF THE STARLINGS (STURNIDAE: STURNINI) AND THE MOCKINGBIRDS (STURNIDAE: MIMINI) CHARLESG. SIBLEYAND JON E. AHLQUIST Departmentof Biologyand PeabodyMuseum of Natural History,Yale University, New Haven, Connecticut 06511 USA ABSTRACT.--OldWorld starlingshave been thought to be related to crowsand their allies, to weaverbirds, or to New World troupials. New World mockingbirdsand thrashershave usually been placed near the thrushesand/or wrens. DNA-DNA hybridization data indi- cated that starlingsand mockingbirdsare more closelyrelated to each other than either is to any other living taxon. Some avian systematistsdoubted this conclusion.Therefore, a more extensiveDNA hybridizationstudy was conducted,and a successfulsearch was made for other evidence of the relationshipbetween starlingsand mockingbirds.The resultssup- port our original conclusionthat the two groupsdiverged from a commonancestor in the late Oligoceneor early Miocene, about 23-28 million yearsago, and that their relationship may be expressedin our passerineclassification, based on DNA comparisons,by placing them as sistertribes in the Family Sturnidae,Superfamily Turdoidea, Parvorder Muscicapae, Suborder Passeres.Their next nearest relatives are the members of the Turdidae, including the typical thrushes,erithacine chats,and muscicapineflycatchers. Received 15 March 1983, acceptedI November1983. STARLINGS are confined to the Old World, dine thrushesinclude Turdus,Catharus, Hylocich- mockingbirdsand thrashersto the New World. la, Zootheraand Myadestes.d) Cinclusis -

Wildland Fire in Ecosystems: Effects of Fire on Fauna
United States Department of Agriculture Wildland Fire in Forest Service Rocky Mountain Ecosystems Research Station General Technical Report RMRS-GTR-42- volume 1 Effects of Fire on Fauna January 2000 Abstract _____________________________________ Smith, Jane Kapler, ed. 2000. Wildland fire in ecosystems: effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 83 p. Fires affect animals mainly through effects on their habitat. Fires often cause short-term increases in wildlife foods that contribute to increases in populations of some animals. These increases are moderated by the animals’ ability to thrive in the altered, often simplified, structure of the postfire environment. The extent of fire effects on animal communities generally depends on the extent of change in habitat structure and species composition caused by fire. Stand-replacement fires usually cause greater changes in the faunal communities of forests than in those of grasslands. Within forests, stand- replacement fires usually alter the animal community more dramatically than understory fires. Animal species are adapted to survive the pattern of fire frequency, season, size, severity, and uniformity that characterized their habitat in presettlement times. When fire frequency increases or decreases substantially or fire severity changes from presettlement patterns, habitat for many animal species declines. Keywords: fire effects, fire management, fire regime, habitat, succession, wildlife The volumes in “The Rainbow Series” will be published during the year 2000. To order, check the box or boxes below, fill in the address form, and send to the mailing address listed below. -

Terrestrial Birds and Conservation Priorities in Baja California Peninsula1
Terrestrial Birds and Conservation Priorities in Baja California Peninsula1 Ricardo Rodríguez-Estrella2 ________________________________________ Abstract The Baja California peninsula has been categorized as as the Nautical Ladder that will have impacts at the an Endemic Bird Area of the world and it is an im- regional level on the biodiversity. Proposals for portant wintering area for a number of aquatic, wading research and conservation action priorities are given for and migratory landbird species. It is an important area the conservation of birds and their habitats throughout for conservation of bird diversity in northwestern the Peninsula of Baja California. México. In spite of this importance, only few, scattered studies have been done on the ecology and biology of bird species, and almost no studies exist for priority relevant species such as endemics, threatened and other key species. The diversity of habitats and climates Introduction permits the great resident landbird species richness throughout the Peninsula, and also explains the pre- The Baja California peninsula is an important area for sence of an important number of landbird migrant conservation of bird diversity in northwestern México species. Approximately 140 resident and 65 migrant (CCA 1999, Arizmendi and Marquez 2000). It has landbird species have been recorded for Baja California been classified as an Endemic Bird Area of the world state (BCN) and 120 resident and 55 landbird migrant (Stattersfield et al. 1998) and also has been considered species for Baja California Sur state (BCS). Three ter- as an important wintering area for a number of aquatic, restrial endemics have been recognized for BCN and wading and migratory landbird species (Massey and four endemics for BCS. -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -

Survey Protocol and Habitat Evaluation for Leconte's
Survey Protocol and Habitat Evaluation for LeConte’s (Toxostoma lecontei) and Bendire’s (Toxostoma bendirei) Thrasher Prepared by: The Desert Thrasher Working Group Survey Protocol and Habitat Evaluation Contents Objective 3 Field Gear and Materials Checklist: 4 Conducting the Survey 6 Thrasher Survey Form 8 Target Species Sighting Form 10 Habitat Evaluation Form 12 Data entry 16 Analysis of Area Search Data: Site-Level Models 17 Appendix 1. Species Descriptions 19 LeConte’s Thrasher 19 Bendire’s Thrasher 20 Appendix 2. Training Materials 22 Appendix 3. Bird and Plant Abbreviations and Codes 25 Appendix 4. Sample Survey Form 33 Appendix 5. Sample Sighting Form 34 Appendix 6. Group Code Examples 35 Appendix 7. Sample Habitat Evaluation Form 37 Appendix 8. Invasive Plant Identification Resources. 38 Recommended Citation: DTWG, the Desert Thrasher Working Group. 2018. Survey Protocol and Habitat Evaluation for LeConte’s and Bendire’sThrashers. The Protocol Subteam with the Desert Thrasher Working Group included: Dawn M. Fletcher, Lauren B. Harter, Christina L. Kondrat-Smith, Christofolos L. McCreedy and Collin A. Woolley. Cover photo art by: Christina Kondrat-Smith 2 Survey Protocol and Habitat Evaluation Objective The objectives of these surveys are to estimate distribution, determine population trends over time, and to identify habitat preferences for Bendire’s and LeConte’s Thrashers. Recommended Survey Times: Consider local elevation and latitude when designing a survey schedule, as researchers will need to balance surveying early (which helps to minimize confusion of adults with juveniles, and which may maximize exposure to peak singing season) with surveying late (which can minimize the possibility of completely missing late-arriving, migratory Bendire’s Thrashers). -

Bird Banding Manual, Vol. II
MTAB 51 July 26, 1983 MEMORANDUM TO : All Banders FROM : Chief, Bird Banding Laboratory Office of Migratory Bird Management Laurel, Maryland 20708 SUBJECT : I. Tabulation of encounters 2. Computer-generated banding schedules 3. Requests for permit revisions 4. Request to not band certain birds 5. BBL slide file needs 6. Permit inactivation 7. A new bride 8. Inland Bird Banding Association meeting I. A tabulation of 1981 banding totals and 1977 banding and recovery totals of species is enclosed. These data are provided primarily to aid in research planning. We may be able to revise this report next year and ask now for suggestions from banders about what data would be most useful to them. 2. Banders may computer-produce banding schedules for the BBL. Any computer- or mini computer-generated schedules (with or without cards or tape) must be BBL-approved in advance of actual schedule submission to the BBL. 3. Please inform all subpermittees that any request for auxiliary marking authorization or changes in permits must come from the master bander. We want requests in writing, please. When requesting permits for a new subpermittee, please furnish the name of the person. We do not send out application forms with the applicant's name blank. 4. Banders are reminded not to use U.S. Fish and Wildlife Service bands on resident game birds (Sp. No. 288 through 31 1.0). Do not band parakeets, rock doves and vultures, or unidentifiable migratory birds such as Empidonax flycatchers, and certain warblers, gulls, and ducks. 5. We would appreciate slides of auxiliary markers from various projects. -
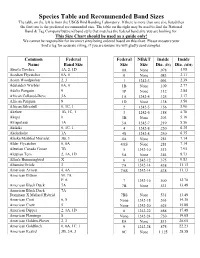
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

Of Bendire's Thrasher
BREEDING HABITAT REQUIREMENTS AND TERRITORY SIZE OF BENDIRE’S THRASHER (Toxostoma bendirei) Photo taken by Cody Bear Sutton Hildago Co, New Mexico Final Report to New Mexico Department of Game & Fish Martha J. Desmond (Principal Investigator) Cody Bear Sutton (Graduate Student) Department of Fish, Wildlife and Conservation Ecology New Mexico State University Las Cruces, NM 880 BREEDING HABITAT REQUIREMENTS AND TERRITORY SIZE OF BENDIRE’S THRASHER (Toxostoma bendirei) TABLE OF CONTENTS INTRODUCTION ..............................................................................................................................3 METHODS ......................................................................................................................................6 Study Area ........................................................................................................................6 Breeding Surveys .............................................................................................................7 Territory Mapping ............................................................................................................9 Abiotic/temporal Measurements ....................................................................................10 Territory Scale Measurements .......................................................................................10 Landscape Scale Measurements .....................................................................................11 DATA ANALYSIS .........................................................................................................................12 -

SAGE THRASHER Oreoscoptes Montanus
A-72 SAGE THRASHER Oreoscoptes montanus Description The sage thrasher is distinguished from other thrashers by its smaller size, short and straight bill, and relatively short tail. Plumage on the upper body is drab, brownish-gray with slightly darker feathers forming indistinct streaks, particularly on the crown. The bird has a pale line behind the ear-coverts, a face pattern formed by whitish supercilium, and a whitish malar region bordered by a black streak at the sides of the throat. The wings are slightly browner than the back and have two narrow white wing-bars. The tail is browner than the rest of the body and has white tipped outer rectrices. The under-parts of the body are off-white, streaked with dark brown blotches. The bill is black with a grayish lower mandible. The plumage remains similar throughout the year but flanks appear pale cinnamon when plumage is fresh in the fall (Reynolds et al. 1999). Life history & Sage thrashers arrive on breeding grounds in March and April (Dillon 1998; behavior Reynolds et al. 1999). Breeding in Colorado typically begins in late May and early June (Dillon 1998). The birds are conspicuous during breeding through An opportunistic activity and song, but are secretive around their nests (Reynolds et al. ground forager and 1999). Adults will fly until they are within 10 meters of the nest and then shrub nester. typically travel on ground the remaining distance. During summer the sage thrasher feeds primarily on ground insects such as ants and beetles, but also feeds on other arthropods, arachnids, plant material, berries and small fruit. -

Birds in a Sagebrush Sea: Managing Sagebrush Habitats for Bird Commu- Nities
BBBIIRRDDSS IINN AA SSAAGGEEBBRRUUSSHH SSEEAA MANAGING SAGEBRUSH HABITATS FOR BIRD COMMUNITIES by Christine Paige and Sharon A. Ritter Partners in Flight, Western Working Group 1999 Research and writing by: Christine Paige, Ravenworks Ecology, 612 Lolo Street, Missoula, MT 59802 Revision and editing by: Sharon A. Ritter, Idaho Partners in Flight, 142 West Hills Way, Hamilton, MT 59840 Editing and layout/design by: Pam Peterson, Nongame Program, Idaho Department of Fish and Game, P.O. Box 25, Boise, ID 83725 Project funding by: USDI Bureau of Land Management, Oregon and Idaho State Offices USDI Fish and Wildlife Service, Region 1 USDA Forest Service, Regions 1 and 4 Idaho Department of Fish and Game, Nongame Program Ravenworks Ecology Acknowledgments: We would like to thank Jack Connelly, Mike Denny, David Dobkin, Randy Hill, Mabel Jankovsky-Jones, Lou Jours, Julie Kaltenecker, Steve Knick, Ron Lambeth, Peter Lessica, Paul Makela, Robert McQuivey, Chris Merker, Bob Moseley, Mike Pellant, Tim Reynolds, Terry Rich, John Rotenberry, Steve Shelly, Michael Schroeder, Chuck Trost, Helen Ulmschneider, Matthew Vander Haegen, and Brian Woodbridge for contributing information and ideas to the development of this document. We owe a special debt to our reviewers for their insightful comments and corrections on the drafts: Loren Anderson, Al Bammann, Jon Beals, Carol Beardmore, Steve Bouffard, Kathy Cheap, Mike Denny, Kim Dickerson, Kristi Dubois, Katy Duffy, Ana Egnew, Jim Hagenbarth, Neil Hedges, Gary Herron, Randy Hill, Nancy Hoffman, Gary Ivey, Bill and Nancy LaFramboise, Ron Lambeth, Tracy Lloyd, Paul Makela, Chris Merker, Alan Peterson, Joe Petzold, Charley Rains, Tim Reynolds, Terry Rich, Bill Roney, John Rotenberry, Michael Schroeder, Dan Svingen, and Helen Ulmschneider. -

North American Important Bird Areas
North American Important Bird Areas A Directory of 150 Key Conservation Sites Table of Contents This publication was prepared by the Secretariat of the Commission for Environmental Cooperation (CEC). The views contained herein do not necessarily reflect the views of the CEC, or the governments of Canada, Mexico or the United States of Table of Contents America. Foreword . v Acknowlegments . ix Reproduction of this document in whole or in part and in any Introduction. 1 form for educational or nonprofit purposes may be made with- Methods. 5 out special permission from the CEC Secretariat, provided Criteria . 9 acknowledgement of the source is made. The CEC would appre- Conservation and Management of Important Bird Areas . 17 How to Read the IBA Site Accounts. 29 ciate receiving a copy of any publication or material that uses this document as a source. Canada . 31 Introduction to the Canadian Sites . 35 Published by the Communications and Public Outreach Depart- United States . 139 ment of the CEC Secretariat. Introduction to the US Sites . 143 For more information about this or other publications from Mexico . 249 the CEC, contact : Introduction to the Mexican Sites. 253 COMMISSION FOR ENVIRONMENTAL COOPERATION 393, rue St-Jacques Ouest, bureau 200 Montréal (Québec) Canada H2Y 1N9 Tel: (514) 350–4300 • Fax: (514) 350–4314 http://www.cec.org ISBN 2-922305-42-2 Disponible en français sous le titre : Les zones importantes pour la con- servation des oiseaux en Amérique du Nord (ISBN 2-922305-44-9). Disponible en español con el título Áreas Importantes para la Conservación de las Aves de América del Norte (ISBN 2-922305-43-0).