Medical Policy Statement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Covered Drugs
List of Covered Drugs Effective July 1, 2016 INTRODUCTION We are pleased to provide the 2011 Gold Coast Health Plan List of Covered Drugs as a useful reference and informational tool. The GCHP List of Covered Drugs can assist practitioners in selecting clinically appropriate and cost effective products for their patients. The information contained in the GCHP List of Covered Drugs is provided solely for the convenience of medical providers. We do not warrant or assure accuracy of such information nor is it intended to be comprehensive in nature. This List of Covered Drugs is not intended to be a substitute for the knowledge, expertise, skill and judgment of the medical provider in his or her choice of prescription drugs. All the information in this List of Covered Drugs is provided as a reference for drug therapy selection. Specific drug selection for an individual patient rests solely with the prescriber. We assume no responsibility for the actions or omissions of any medical provider based upon reliance, in whole or in part, on the information contained herein. The medical provider should consult the drug manufacturer's product literature or standard references for more detailed information. National guidelines can be found on the National Guideline Clearinghouse site at http://www.guideline.gov PREFACE The GCHP List of Covered Drugs is organized by sections. Each section is divided by therapeutic drug class primarily defined by mechanism of action. Unless exceptions are noted, generally all applicable dosage forms and strengths of the drug cited are included in the GCHP List of Covered Drugs. Generics should be considered the first line of prescribing. -

Cerezyme, INN- Imiglucerase
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Cerezyme. This scientific discussion has been updated until 01 August 2003. For information on changes after this date please refer to module 8B. 1. Introduction Gaucher disease, also called glucosylceramide lipidosis or ß-glucocerebrosidase (GCR) deficiency, is the most common of the sphingolipidoses or lipid storage diseases, a group of diseases that are inherited in an autosomal recessive manner. It is characterised by the accumulation of glucocerebroside in tissue macrophages. Gaucher disease presents in three subtypes: type 1, the non- neuronopathic form; type 2, the acute neuronopathic form; and 3, the chronic neuronopathic form. Of these, type 1, non-neuronopathic form, is the most frequent and type 2, acute neuronopathic, is the least frequent. It has been estimated that type 1 Gaucher disease affects more than 20,000 patients world-wide. The clinical features of Gaucher disease include anaemia and thrombocytopenia due to splenic sequestration and bone marrow replacement by accumulating Gaucher cells. Splenomegaly and hepatomegaly are other frequent signs. Skeletal disease eventually leading to pain, stress fractures and osteonecrosis are common symptoms in type 1 and 3 Gaucher disease. The neuronopathic forms also present neurological abnormalities such as seizures, dementia, spasticity, ataxia and loss of intellectual functions. Enzyme replacement therapy (ERT) of Gaucher disease has been made feasible by the introduction of mannose-terminated ß-glucocerebrosidase. Mannose is a sugar, which is abundant on the surfaces of micro-organisms. It may be bound by the mannose receptor of macrophages. The mannose- termination of ß-glucocerebrosidase leads to a selective uptake of the enzyme by macrophages that are present in liver, spleen and skeleton. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -
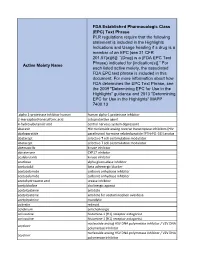
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Miglustat (NB-DNJ)
Article pubs.acs.org/molecularpharmaceutics Therapeutic Strategies for Gaucher Disease: Miglustat (NB-DNJ) as a Pharmacological Chaperone for Glucocerebrosidase and the Different Thermostability of Velaglucerase Alfa and Imiglucerase Olga Abian,*, ,!,§,% Pilar Alfonso,*, ,!,¥ Adrian Velazquez-Campoy,§,#,∇ Pilar Giraldo, ,!,¥,Ë Miguel Pocovi,!,¥,# and Javier Sancho§,# Unidad de Investigación Traslacional, Miguel Servet Universitary Hospital, Zaragoza, Spain !Aragon Health Sciences Institute (I+CS), Zaragoza, Spain §Institute of Biocomputation and Physics of Complex Systems (BIFI), University of Zaragoza, BIFI-IQFR (CSIC)-Joint Unit, Spain %Centro de Investigación Biomedicá en Red en el Área Tematicá de Enfermedades Hepaticaś y Digestivas (CIBERehd) and ¥Centro de Investigación en Red de Enfermedades Raras (CIBERER), ISCIII, Spain #Department of Biochemistry and Cellular and Molecular Biology, University of Zaragoza, Spain ∇Fundación ARAID (ARAID-BIFI), Diputación General de Aragón, Spain ËService of Hematology, Miguel Servet Universitary Hospital, Zaragoza, Spain ABSTRACT: Gaucher disease (GD) is a disorder of glycosphingolipid metabolism caused by deficiency of lysosomal glucocerebrosidase (GlcCerase) activity, due to conformationally or functionally defective variants, resulting in progressive deposition of glycosylceramide in macrophages. The glucose analogue, N-butyldeoxynojirimycin (NB- DNJ, miglustat), is an inhibitor of the ceramide-specific glycosyltransfer- ase, which catalyzes the first step of glycosphingolipid biosynthesis -

Quantum of Effectiveness Evidence in FDA's Approval of Orphan Drugs
Quantum of Effectiveness Evidence in FDA’s Approval of Orphan Drugs Cataloguing FDA’s Flexibility in Regulating Therapies for Persons with Rare Disorders by Frank J. Sasinowski, M.S., M.P.H., J.D.1 Chairman of the Board National Organization for Rare Disorders One of the key underlying issues facing the development of effective for their intended uses. all drugs, and particularly orphan drugs, is what kind of evi- dence the Food and Drug Administration (FDA) requires for FDA has for many decades acknowledged that there is a need approval. The Federal Food, Drug, and Cosmetic [FD&C] Act for flexibility in applying its standard for approval. For ex- provides that for FDA to grant approval for a new drug, there ample, one of FDA’s regulations states that: “FDA will ap- must be “substantial evidence” of effectiveness derived from prove an application after it determines that the drug meets the “adequate and well-controlled investigations.” This language, statutory standards for safety and effectiveness… While the which dates from 1962, provides leeway for FDA medical re- statutory standards apply to all drugs, the many kinds of drugs viewers to make judgments as to what constitutes “substantial that are subject to the statutory standards and the wide range evidence” of a drug’s effectiveness, that is, of its benefit to of uses for those drugs demand flexibility in applying the stan- patients. dards. Thus FDA is required to exercise its scientific judgment to determine the kind and quantity of data and information an The sole law that applies specifically to orphan drugs, the Or- applicant is required to provide for a particular drug to meet phan Drug Act of 1983, provided financial incentives for drug the statutory standards.” 21 C.F.R. -

For Gaucher Disease
NINDS Contributions to Approved Therapies NINDS invests in and conducts research across the spectrum of neuroscience and neurology research, from basic studies on fundamental biological mechanisms, to clinical trials to test new treatments in patients. Here, we describe the path leading to the development and approval of one therapy for a neurological disorder, and we highlight contributions enabled by NINDS and NIH support. Alglucerase Injection (Ceredase®) for Gaucher Disease Overview Lysosomes are compartments in cells that Gaucher disease, a severe disorder with dispose and recycle waste, using specific onset in infancy or before birth, and Type enzymes to break down cellular debris 3 Gaucher disease, a milder form with into components that can be reused or later childhood or adult onset. discarded. People with lysosomal storage disorders lack one or more of these Physician-scientist Roscoe O. Brady enzymes, causing certain waste products (1923-2016) was an intramural investiga- to accumulate in cells and resulting in a tor at NINDS who dedicated much of his variety of symptoms. In Gaucher disease, career to research on lysosomal storage the deficient enzyme is glucocerebro- disorders. His work to identify the missing sidase, which degrades the fatty molecule enzyme in Gaucher disease and determine glucocerebroside (also called glucosylcer- how to restore it ultimately led to the amide). Type 1 Gaucher disease primarily development of alglucerase injection affects macrophages, a type of white (Ceredase®, Genzyme) for the treatment blood cell that devours other worn-out of Type 1 Gaucher disease, the first cells. Symptoms range from mild to severe enzyme replacement therapy approved and include liver and spleen enlargement, in the U.S. -

(INN) for Biological and Biotechnological Substances
INN Working Document 05.179 Update 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: Active Moiety Name “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. -

Drug Prior Authorization List
Drug Prior Authorization List Why do some drugs require prior authorization? Prior authorization is a tool to ensure the appropriate use of certain drugs and allows us to determine if a drug meets the medical necessity requirements of your policy. Who makes the prior authorization decisions? Physicians and pharmacists at your health plan or at one of our partners, Diplomat or Express Scripts. The list to follow specifies who performs the review and makes the decision. Why am I sometimes asked to use a different drug than my doctor prescribed? If you go to the pharmacy to have your prescription filled before getting prior authorization when required, your pharmacist may tell you about other medications that may be equally effective but don’t require prior authorization. If this occurs, contact your doctor to ask about changing the prescription to the other drug. If your doctor approves, the pharmacy can immediately fill the prescription. What information is used by the physician or pharmacist in the decision-making process? Medical records describing the patient’s condition and prior treatments, FDA approved labeling for the requested treatment, published and peer-reviewed scientific literature, and/or evidence-based guidelines. Where can I view or obtain a copy of the prior authorization or step therapy criteria? l For drugs reviewed by Diplomat, you can call 1-888-515-1357 or access the current prior authorization criteria online at www.diplomatpharmacy.com/criteria. l For drugs reviewed by Express Scripts, please call 1-800-753-2851 to speak with a prior authorization specialist for more detailed information. -

Federal and Private Roles in the Development and Provision of Alglucerase Therapy for Gaucher Disease
Federal and Private Roles in the Development and Provision of Alglucerase Therapy for Gaucher Disease October 1992 OTA-BP-H-104 NTIS order #PB93-101723 For sale by the U.S. Government Printing Office Superintendent of Documents, Mail Stop:SSOP, Washington, DC 20402-9328 ISBN 0-16-038129-0 Foreword The effort to discover and develop new pharmaceuticals is a risky and costly enterprise. For diseases that affect few patients, the barriers to development maybe especially great, since the drugs’ small markets may make it difficult for firms to recoup their initial research and development investments. The Federal Government has sought to reduce these barriers through incentives first adopted in the Orphan Drug Act of 1983 (Public Law 97-414). The transfer of technology from Federal laboratories such as the National Institutes of Health to the pharmaceutical industry can also reduce the cost and risk of drug development for firms. Although such incentives may result in important new therapies, their price to patients and insurers may still be high. As part of our assessment, Government Policies and Pharmaceutical Research and Development, requested by the House Committee on Energy and Commerce and its Subcommittee on Health and the Environment and the Subcommittee on Antitrust, Monopolies, and Business Rights of the Senate Committee on the Judiciary, OTA commissioned researchers at Stanford University to examine the development and provision of alglucerase, an important new treatment for Gaucher disease. Gaucher disease is a rare inherited disorder in which the body lacks an enzyme necessary to break down fats. This background paper describes the development of alglucerase, illustrates the role that both the Federal Government and private sector can have in making new therapies available for orphan diseases, and lays out some of the tradeoffs that can exist between developing new medical technologies and controlling health care costs. -

FDA Listing of Established Pharmacologic Class Text Phrases January 2021
FDA Listing of Established Pharmacologic Class Text Phrases January 2021 FDA EPC Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for Active Moiety Name [indication(s)].” For each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13.