10. Committee Section (H&Fw)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
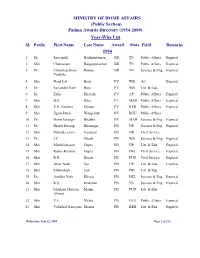
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

S.No. Sector Core Group Constituted?
APPENDIX I List of Sectors S.No. Sector Core Group Constituted? 1 Automobile Yes 2 Beauty and Wellness Yes 3 Construction, Construction Material, and Real Estate Yes 4 Electronics & Hardware Yes 5 Fabrication Yes 6 Food Processing & Preservation Yes 7 IT & ITES Yes 8 Power Generation, Transmission, Distribution, Wiring, and Yes Electrical Equipments 9 Production & Manufacturing Yes 10 Textiles and Apparel Yes 11 Travel, Tourism, and Hospitality Yes 12 Organized Retail No 13 Telecommunications No 14 Industrial Automation & Instrumentation No 15 Handicrafts No 16 Mining & Mineral Processing No 17 Plastics No 18 Leather & Leather Goods No 19 Gems And Jewellery No 20 Agriculture, Horticulture, Floriculture No 21 Dairy, Meat, Poultry, Fisheries No 22 Rubber No 23 Health Care No 24 Entertainment, Media, Advertising, Event Management No 25 Banking Financial Service & Insurance (BFSI) No ANNEXURE I List of Sectors S.No. Sector 1 Automobile 2 Beauty and Wellness 3 Construction, Construction Material, and Real Estate 4 Electronics & Hardware 5 Fabrication 6 Food Processing & Preservation 7 IT & ITES 8 Power Generation, Transmission, Distribution, Wiring, and Electrical Equipments 9 Production & Manufacturing 10 Textiles and Apparel 11 Travel, Tourism, and Hospitality ANNEXURE II CORE GROUP FOR AUTOMOBILE SECTOR S.No. Name and Designation Contribution in Core Group 1 Shri T.C. Saravanabava, DDG(AT), DGET Mentor Headquarters 2 Mr. K.S. Rao, JDT, CSTARI, Kolkata Representative of CSTARI 3 Mr. Ravichandran, DDT, NIMI Chennai Representative of NIMI 4 Mr. R.K. Gowda, DDT, ATI Hyderabad Champion Master Trainer 5 Mr. Yuvraj, DDT, ATI Chennai Member 6 Mr. G. Venktesh, ADT, ATI Hyderabad Member 7 Mr. -

King's Research Portal
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by King's Research Portal King’s Research Portal DOI: 10.1016/j.healthplace.2016.08.004 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Murray, S. F., Bisht, R., & Pitchforth, E. (2016). Emplacing India's “medicities”. HEALTH AND PLACE, 42, 69-78. https://doi.org/10.1016/j.healthplace.2016.08.004 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Emplacing India's Medicities MURRAY Firstonline28september2016 GREEN
King’s Research Portal DOI: 10.1016/j.healthplace.2016.08.004 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Murray, S. F., Bisht, R., & Pitchforth, E. (2016). Emplacing India's “medicities”. HEALTH AND PLACE, 42, 69-78. https://doi.org/10.1016/j.healthplace.2016.08.004 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Report on Mapping of Healthcare Sector in India
Report on Mapping of Healthcare Sector in India SWECARE AND SWEDISH TRADE COUNCIL, INDIA 2012 This page has been intentionally left blank Page 2 of 294 Table of Contents 1. EXECUTIVE SUMMARY ......................................................................................................................... 8 1.1. OVERVIEW - HEALTH SITUATION IN INDIA .................................................................................................... 8 1.2. SHORTLISTED SECTORS AND MAJOR BUSINESS OPPORTUNITIES............................................................... 11 1.3. PERCEPTION REGARDING SWEDISH TECHNOLOGIES AND SOLUTIONS ........................................................ 22 2. HEALTHCARE SECTOR IN INDIA ....................................................................................................... 24 2.1. OVERVIEW ............................................................................................................................................. 24 3. MAJOR HEALTHCARE PROGRAMS .................................................................................................. 33 3.1. NATIONAL RURAL HEALTH MISSION ......................................................................................................... 34 3.2. NATIONAL URBAN HEALTH MISSION ......................................................................................................... 39 3.3. NATIONAL VECTOR BORNE DISEASE CONTROL PROGRAM ........................................................................ 42 3.4. REVISED -

Dr. Devi Shetty - Doctor of Science
Dr. Devi Shetty - Doctor of Science Dr. Devi Shetty, Chairman, Narayana Health Group was awarded today with an honorary doctorate degree of Doctor of Science for his contributions and commitment to the field of Medical Science. The Doctorate was conferred upon him with the consent of the President of India Pranab Mukherjee to the proposal made by Indian Institute of Technology Madras (IIT-M). The Honorary Degree was bestowed by Prof. Bhaskar Rammurthi – Director, IIT- M as part of 51st convocation at a ceremony in Chennai. Receiving the award, Dr. Devi Shetty, Chairman, Narayana Health, said, A tribute to a person is always an acknowledgment of his ideologies, efforts and the purpose to which that person has devoted his life. It has been our constant endeavor at Narayana Health to bring down the cost of healthcare and make quality healthcare accessible to all sections of society. Thus, I share this award with all those who have also dedicated themselves for this cause. He also added, Consolidation is the only way to reach out to a larger level, for which it s important to partner with multiple stakeholders, including academia. Dr. Devi Shetty is also the recipient of the prestigious civilian award, Padma Bhushan for his contributions in the field of healthcare, especially heart care After completing his graduate degree in Medicine and post-graduate work in General Surgery from Kasturba Medical College, Mangalore, he trained in cardiac surgery at Guys Hospital in the United Kingdom. He performed the first neonatal heart surgery in the country on a 9-day-old baby. -

Bangalore Faculty of Art and Design
List of Alumni of M. S. Ramaiah University of Applied Sciences (SAMPARK), Bangalore Faculty of Art and Design Sl. Programme Year of Name Contact Address Photograph e-mail id No. Completed Admission Mobile no. 650 M. Des. (Product 2015 E SUMANTH H NO.43/233, N R 7075990455 Design) PET, KURNOOL, SUMANTHZ ANDHRA PRADESH- @LIVE.IN 518004 649 M. Des. (Product 2015 JAYANT G 27,"SHREE 8147999872 Design) ANKOLEKAR SHARVARI, C- jayantga@y BLOCK, APOORVA ahoo.in NAGAR, GOKUL ROAD, HUBLI- 580030 648 M. Des. (Product 2015 PRANATI AMITHORE 9609951821 Design) MAHANTY VILLAGE, 09199312p SUKHADALLI POST, [email protected] ANKURA DISTRICT, m WEST BENGAL- 722150 647 M. Des. (Product 2015 SACHIN 6586, GUBBI 9036075886 Design) JAYAPRAKASH HUCHAPPA sachinjayapr LAYOUT, SUBHASH akash26@g NAGAR, mail.com NELAMANGALA- 562123 646 M. Des. (Product 2015 SHASHANK U K 5, 5TH CROSS, 80FT 8951273001 Design) ROAD, shashankuk MAHALAKSHMI [email protected] NAGAR, BATWADI, om TUMKUR-572103 645 M. Des. (Product 2015 ROSHITH K P RESHMALAUYAM 9596164633 Design) (H),VATAKARA, designs.spe KOZHIKODE, [email protected] KERALA-673104 om 644 M. Des. (Product 2015 GOVARTHANAM 23, OLD BAZER 9566752210 Design) V STREET, TIRUTANI, govaa082@ TIRUVALLUR gmail.com DISTRICT, TAMIL NADU-631209 List of Alumni of M. S. Ramaiah University of Applied Sciences (SAMPARK), Bangalore Faculty of Art and Design Sl. No. Programme Year of Name Contact Address Photograph e-mail id/Mobile no. Completed Admission 643 M.Des CAGD 2014 Arjun Nedungadi SWAPNAM HOUSE, NO-94, OPP. 9633940649 KENDRIYA VIDYALAYA, KADAVANTHRA. KOCHI-682020 -

Download Here the Issue Dated June 5
V OLUME 37 NUMBER 11 MAY 23-JUNE05, 2020 ISSN 0970-1710 HTTPS://FRONTLINE.THEHINDU.COM C OVER STORY Gujarat: Cavalier approach 66 Kerala: New worries 68 How not to handle an epidemic Karnataka: Migrant misery 71 Odisha: Sarpanchs as The lockdowns were meant to buy time to contain the coronavirus’ game changers 73 spread, but they have instead heaped misery on the marginalised. Tamil Nadu: Chennai a challenge 76 India is still in the exponential phase of the infection and community Jammu & Kashmir: transmission is a reality that the government refuses to accept. 4 Viral politics 78 Violence unabated in Kashmir Valley 80 Hate in the time of pandemic 82 Urban planning’s disregard of migrants 85 Scapegoating China 88 Unemployment crisis in the U.S. 92 ENVIRONMENT Maheshwar dam: Victory and vindication 94 A n empty package 8 Questions over role of D elhi: Capital unconcern 46 AGRICULTURE Supreme Court 30 Uttar Pradesh: Losing the plot 48 Rajasthan: Squandered gains 50 Haryana: In denial mode 52 Bihar: Community clout 54 Madhya Pradesh: Migrants’ ire 56 P unjab: A Bill to Signs of dissent in BJP 14 Ekta Parishad survey: Chhattisgarh: An ‘corporatise’ farming 97 No love lost for labour 17 Understanding migration 35 oasis of comfort 58 COVID response Tragedy on foot Jharkhand: Cashless lacks a strategy 20 in Tamil Nadu 39 and abandoned 60 Capital’s Malthusian Stranded in foreign soil 42 West Bengal: Caught moment 23 in politics 62 States trapped in a fiscal STATES Maharashtra: Stretched and developmental crisis 27 P unjab: Feeling the pinch 44 resources 64 On the Cover: Inside a container truck near Hyderabad, migrants hoping to make it to their villages in Uttar Pradesh, on May 12. -

Project Closure Report
KJA RECOMMENDATION (7th KJA MEETING ON SEP 7, 2017) Project Closure Report N. Sreekanth Nayak, Pavan Sridharan and B Gurumoorthy, Centre for Product Design and Manufacturing, IISc 1 Contents Acknowledgements ....................................................................................................................................... 5 Executive Summary ....................................................................................................................................... 6 1. Introduction .............................................................................................................................................. 7 i. Background .................................................................................................................................................................. 7 ii. Emergence of the NTS Concept ............................................................................................................................ 7 iii. Design and Development of the NTS System.................................................................................................. 7 iv. Structure of the NTS.................................................................................................................................................. 8 2. Content and Scenario Structure .............................................................................................................. 11 i. Learning Objectives of Simulation for Nurses ........................................................................................... -

Alphabetical List of Recommendations Received for Padma Awards - 2014
Alphabetical List of recommendations received for Padma Awards - 2014 Sl. No. Name Recommending Authority 1. Shri Manoj Tibrewal Aakash Shri Sriprakash Jaiswal, Minister of Coal, Govt. of India. 2. Dr. (Smt.) Durga Pathak Aarti 1.Dr. Raman Singh, Chief Minister, Govt. of Chhattisgarh. 2.Shri Madhusudan Yadav, MP, Lok Sabha. 3.Shri Motilal Vora, MP, Rajya Sabha. 4.Shri Nand Kumar Saay, MP, Rajya Sabha. 5.Shri Nirmal Kumar Richhariya, Raipur, Chhattisgarh. 6.Shri N.K. Richarya, Chhattisgarh. 3. Dr. Naheed Abidi Dr. Karan Singh, MP, Rajya Sabha & Padma Vibhushan awardee. 4. Dr. Thomas Abraham Shri Inder Singh, Chairman, Global Organization of People Indian Origin, USA. 5. Dr. Yash Pal Abrol Prof. M.S. Swaminathan, Padma Vibhushan awardee. 6. Shri S.K. Acharigi Self 7. Dr. Subrat Kumar Acharya Padma Award Committee. 8. Shri Achintya Kumar Acharya Self 9. Dr. Hariram Acharya Government of Rajasthan. 10. Guru Shashadhar Acharya Ministry of Culture, Govt. of India. 11. Shri Somnath Adhikary Self 12. Dr. Sunkara Venkata Adinarayana Rao Shri Ganta Srinivasa Rao, Minister for Infrastructure & Investments, Ports, Airporst & Natural Gas, Govt. of Andhra Pradesh. 13. Prof. S.H. Advani Dr. S.K. Rana, Consultant Cardiologist & Physician, Kolkata. 14. Shri Vikas Agarwal Self 15. Prof. Amar Agarwal Shri M. Anandan, MP, Lok Sabha. 16. Shri Apoorv Agarwal 1.Shri Praveen Singh Aron, MP, Lok Sabha. 2.Dr. Arun Kumar Saxena, MLA, Uttar Pradesh. 17. Shri Uttam Prakash Agarwal Dr. Deepak K. Tempe, Dean, Maulana Azad Medical College. 18. Dr. Shekhar Agarwal 1.Dr. Ashok Kumar Walia, Minister of Health & Family Welfare, Higher Education & TTE, Skill Mission/Labour, Irrigation & Floods Control, Govt. -

International Coronary Congress 2016
INTERNATIONAL CORONARY Medanta Institute of Education and Research CONGRESS presents 2016 November 11 - 13, 2016 Hotel Taj Palace New Delhi, India State of the Art Course of Coronary Revascularization in association with IACTS & APSIC Indian Association of Asian Pacific Society of Cardiovascular-Thoracic Surgeons Interventional Cardiolog First ever Heart Team Congress on Coronary Revascularization Scientific Programme rge DIC Su on ME AL of s C o A O e f N U g E A e N l d Y l C i R o 11.5 CPD n 20 Credit I A L b C u H l r a Point HOURS g y h o R RCS CPD points are recognised the world over, including in the US and Europe The possibilities are endless when a cardiologist and a cardiac surgeon come together to treat Coronary Artery Disease A world with the purest of meritocracies of Coronary Artery Disease Invitation International Coronary Congress 2016 Dear Friends & Colleagues, It is my privilege to welcome you to the International Coronary Congress 2016, New Delhi, India . AT ICC 2016 we are determined to make the first of its kind comprehensive course on coronary artery disease Dr. Naresh Trehan management where the platform is shared by most eminent cardiac surgeons and cardiologists from the world to give you a complete overview and meet all your unmet needs. The time has come when we have to introspect, leave the controversies and egos behind, and changes our mindset of individualistic approach of coronary artery disease management to more consensus -based approaches. In the light of evidences from recent trials its proved that HEART TEAM is the way forward in the management of cardiovascular diseases be it coronary artery disease or structural heart disease. -

National Affairs
NATIONAL AFFAIRS Prithvi II Missile Successfully Testifi ed India on November 19, 2006 successfully test-fi red the nuclear-capsule airforce version of the surface-to- surface Prithvi II missile from a defence base in Orissa. It is designed for battlefi eld use agaisnt troops or armoured formations. India-China Relations China’s President Hu Jintao arrived in India on November 20, 2006 on a fourday visit that was aimed at consolidating trade and bilateral cooperation as well as ending years of mistrust between the two Asian giants. Hu, the fi rst Chinese head of state to visit India in more than a decade, was received at the airport in New Delhi by India’s Foreign Minister Pranab Mukherjee and Science and Technology Minister Kapil Sibal. The Chinese leader held talks with Indian Prime Minister Manmohan Singh in Delhi on a range of bilateral issues, including commercial and economic cooperation. The two also reviewed progress in resolving the protracted border dispute between the two countries. After the summit, India and China signed various pacts in areas such as trade, economics, health and education and added “more substance” to their strategic partnership in the context of the evolving global order. India and China signed as many as 13 bilateral agreements in the presence of visiting Chinese President Hu Jintao and Prime Minister Manmohan Singh. The fi rst three were signed by External Affairs Minister Pranab Mukherjee and Chinese Foreign Minister Li Zhaoxing. They are: (1) Protocol on the establishment of Consulates-General at Guangzhou and Kolkata. It provides for an Indian Consulate- General in Guangzhou with its consular district covering seven Chinese provinces of Guangdong, Fujian, Hunan, Hainan, Yunnan, Sichuan and Guangxi Zhuang autonomous region.