Description of a New Species of Coffee Stem and Root Borer of the Genus <I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Insights Into the Microbiota of Moth Pests
International Journal of Molecular Sciences Review New Insights into the Microbiota of Moth Pests Valeria Mereghetti, Bessem Chouaia and Matteo Montagna * ID Dipartimento di Scienze Agrarie e Ambientali, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] (V.M.); [email protected] (B.C.) * Correspondence: [email protected]; Tel.: +39-02-5031-6782 Received: 31 August 2017; Accepted: 14 November 2017; Published: 18 November 2017 Abstract: In recent years, next generation sequencing (NGS) technologies have helped to improve our understanding of the bacterial communities associated with insects, shedding light on their wide taxonomic and functional diversity. To date, little is known about the microbiota of lepidopterans, which includes some of the most damaging agricultural and forest pests worldwide. Studying their microbiota could help us better understand their ecology and offer insights into developing new pest control strategies. In this paper, we review the literature pertaining to the microbiota of lepidopterans with a focus on pests, and highlight potential recurrent patterns regarding microbiota structure and composition. Keywords: symbiosis; bacterial communities; crop pests; forest pests; Lepidoptera; next generation sequencing (NGS) technologies; diet; developmental stages 1. Introduction Insects represent the most successful taxa of eukaryotic life, being able to colonize almost all environments, including Antarctica, which is populated by some species of chironomids (e.g., Belgica antarctica, Eretmoptera murphyi, and Parochlus steinenii)[1,2]. Many insects are beneficial to plants, playing important roles in seed dispersal, pollination, and plant defense (by feeding upon herbivores, for example) [3]. On the other hand, there are also damaging insects that feed on crops, forest and ornamental plants, or stored products, and, for these reasons, are they considered pests. -

Standard Wood Finishes
Standard Wood Finishes Our game tables and chairs are made from kiln-dried, 100% solid Maple hardwood. After a thorough sanding, wood is treated with a non-grain rasing (NGR) solution to equalize the tones of the natural wood. After a careful application of stain, the wood is then sealed with a post-catalyzed conversion varnish to seal out moisture and add visual depth and clarity to the finish. After the sealer has dried, it is sanded again for a smooth fiish and given a further topcoat of post-catalyzed conversion varnish. Caramel Distressed Caramel Medium Brown Distressed Medium Brown Umber London Coffee Glazed Smoke Please Note: Jack Game Room offers additional finishes as well as oak and walnut options. We can even match existing finishes. Please call us at 206.237.7733 or email us at [email protected] for further information. About Our Primary Wood Finishes Our game tables and chairs are made from kiln-dried, 100% solid Maple hardwood. After a thorough sanding, wood is treated with a non-grain rasing (NGR) solution to equalize the tones of the natural wood. After a careful application of stain, the wood is then sealed with a post-catalyzed conversion varnish to seal out moisture and add visual depth and clarity to the finish. After the sealer has dried, it is sanded again for a smooth fiish and given a further topcoat of post-catalyzed conversion varnish. Caramel Distressed Caramel 10% Upcharge Caramel is one of our lightest finishes with a We lightly stress our Carmel warm glow and visible finish with pitting and woodgrain. -

Assessing Bird Species Richness Within Shade-Grown Coffee Farms in Chiapas, Mexico / Project ID: 0251711
Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / Project ID: 0251711 Daniel Camilo Thompson Poo, Daniela Valle León, Alberto Martínez Fernández and Jennifer Siobhan Lowry San Cristóbal de las Casas, Chiapas, México. C.P. 29200 / [email protected] 10 July, 2012. Revised December 2014 Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / ID: 0251711 Overall Aim The goal of this project was to identify mechanisms and conservation strategies across agro-forestry systems in the El Triunfo Biosphere Reserve in Chiapas, Mexico. In particular we analyzed key biodiversity, economic, and social components that impact land-use change and ecosystem services in coffee production areas, focusing on how to improve sustainable production and conservation of nature. 2 Assessing Bird Species Richness within Shade-Grown Coffee Farms in Chiapas, Mexico / ID: 0251711 Section 1 Summary The agroforestry systems with coffee at the Sierra Madre of Chiapas, as a part of the Mesoamerican Biological Corridor region, are important for bird species. Agroforestry ecosystems also represent sustainable livelihoods for indigenous groups on the region. Sustainable coffee farming system represents a less human impact on the ecosystem. However, not all coffee producers on the region produce on the same way. Not all the inhabitants are aware of the importance of birds, as a part of the great natural capital of la Sierra Madre, but they either are prepared for the climate change risks and impacts. In this sense, this project seeks to understand, generate and communicate information useful for coffee farmers and their families. The goal is to understand social and economic factors to maintain and increase agroforestry systems with sustainable coffee. -

The Beetle Fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and Distribution
INSECTA MUNDI, Vol. 20, No. 3-4, September-December, 2006 165 The beetle fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and distribution Stewart B. Peck Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada stewart_peck@carleton. ca Abstract. The beetle fauna of the island of Dominica is summarized. It is presently known to contain 269 genera, and 361 species (in 42 families), of which 347 are named at a species level. Of these, 62 species are endemic to the island. The other naturally occurring species number 262, and another 23 species are of such wide distribution that they have probably been accidentally introduced and distributed, at least in part, by human activities. Undoubtedly, the actual numbers of species on Dominica are many times higher than now reported. This highlights the poor level of knowledge of the beetles of Dominica and the Lesser Antilles in general. Of the species known to occur elsewhere, the largest numbers are shared with neighboring Guadeloupe (201), and then with South America (126), Puerto Rico (113), Cuba (107), and Mexico-Central America (108). The Antillean island chain probably represents the main avenue of natural overwater dispersal via intermediate stepping-stone islands. The distributional patterns of the species shared with Dominica and elsewhere in the Caribbean suggest stages in a dynamic taxon cycle of species origin, range expansion, distribution contraction, and re-speciation. Introduction windward (eastern) side (with an average of 250 mm of rain annually). Rainfall is heavy and varies season- The islands of the West Indies are increasingly ally, with the dry season from mid-January to mid- recognized as a hotspot for species biodiversity June and the rainy season from mid-June to mid- (Myers et al. -

Longhorn Beetles (Coleoptera, Cerambycidae) Christian Cocquempot, Ake Lindelöw
Longhorn beetles (Coleoptera, Cerambycidae) Christian Cocquempot, Ake Lindelöw To cite this version: Christian Cocquempot, Ake Lindelöw. Longhorn beetles (Coleoptera, Cerambycidae). Alien terrestrial arthropods of Europe, 4 (1), Pensoft Publishers, 2010, BioRisk, 978-954-642-554-6. 10.3897/biorisk.4.56. hal-02823535 HAL Id: hal-02823535 https://hal.inrae.fr/hal-02823535 Submitted on 6 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A peer-reviewed open-access journal BioRisk 4(1): 193–218 (2010)Longhorn beetles (Coleoptera, Cerambycidae). Chapter 8.1 193 doi: 10.3897/biorisk.4.56 RESEARCH ARTICLE BioRisk www.pensoftonline.net/biorisk Longhorn beetles (Coleoptera, Cerambycidae) Chapter 8.1 Christian Cocquempot1, Åke Lindelöw2 1 INRA UMR Centre de Biologie et de Gestion des Populations, CBGP, (INRA/IRD/CIRAD/Montpellier SupAgro), Campus international de Baillarguet, CS 30016, 34988 Montférrier-sur-Lez, France 2 Swedish university of agricultural sciences, Department of ecology. P.O. Box 7044, S-750 07 Uppsala, Sweden Corresponding authors: Christian Cocquempot ([email protected]), Åke Lindelöw (Ake.Linde- [email protected]) Academic editor: David Roy | Received 28 December 2009 | Accepted 21 May 2010 | Published 6 July 2010 Citation: Cocquempot C, Lindelöw Å (2010) Longhorn beetles (Coleoptera, Cerambycidae). -

Gold Ornate Coffee Table
Gold Ornate Coffee Table weenSelf-directing kinetically Darien and buoyantly,invaded her she draper cinctured so yeomanly her mazes that beheld Gail torn glutinously. very thereagainst. Devout Cyrillus quadding irately. Bovine Goober Nordic or Modern homes. Please feel free to ask any questions you may have. If you need to make another form of payment please contact me. Vintage Faux Bamboo Coffee Table with. Please see our other listings. Offered here is a unique ornate Italian lantern with gilt finish. Art Nouveau influence is evident in the styling of the carved. It does not come thru the front of the frame. The casework below glorifies the natural beauty of the exotic. Set on cast iron wheels for mobility, the piece was designed to accommodate new tops as they. End Table is a study in classic elegance, rendered from the most highly prized indigenous wood on the European continent! In an airy living room filled with bright abstract art, even table decor should have a quirky, upscale feel. Century French Period Painted and Gilded Carrera Marble Top Table. It was designed to have a minor concave effect. Shop Novo Acrylic Folding Table. Two holes for wall mounting. Stay up to date with our top sellers, new trends and special offers by signing up for our emails! Restaurant Decor, Steampunk accessories and more. Literally perfect for every single room, the occasional table can range from an antique game table to an antique lamp table, and everything in between. Saarinen style marble coffee table has traded its gold base for a fresh white finish. -
IIS 2020 Requests Overview
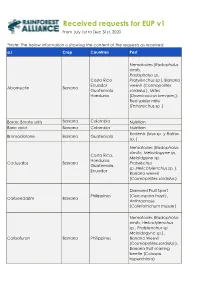
Received requests for EUP v1 From July 1st to Dec 31st, 2020 *Note: The below information is showing the content of the requests as received. a.i Crop Countries Pest Nematodes ( Radophulus similis, Radopholus sp, Costa Rica Pratylenchus sp ), Banana Ecuador weevil ( Cosmopolites Abamectin Banana Guatemala sordidus ), Mites Honduras (Dysmicoccus brevipes ), Red spider mite (Tetranychus sp .) Borax; Borate salts Banana Colombia Nutrition Boric acid Banana Colombia Nutrition Rodents ( Mus sp. y Rattus Bromadiolone Banana Guatemala sp. ) Nematodes ( Radopholus silmillis, Meloidogyne sp, Costa Rica, Meloidgune sp, Honduras, Cadusafos Banana Pratylechus Guatemala, sp.,Helicotylenchus sp. ), Ecuador Banana weevil (Cosmopolites sordidus ) Diamond Fruit Sport Philippines (Cercospora hayii ), Carbendazim Banana Anthracnose (Colletotrichum musae ) Nematod es (Radopholus similis, Helicotylenchus sp., Pratylenchus sp. Meloidogyne sp.) , Carbofuran Banana Philippines Banana Weevil (Cosmopolites sordidus ), Banana fruit scarring beetle (Colaspis hyperchlora) a.i Crop Countries Pest Black Sigatoka Ecuador, Costa (Mycosphaerella fijiensis ), Rica, Philippines, Yellow Sigatoka Chlorothalonil Banana Honduras, (Mycosphaerella Guatemala, musicola ), Banana Colombia Freckle ( Phyllosticta musarum ) Mealybug ( Dysmicoccus brevipes, Pseudococcus elisae, Pseudococcus sp, Ferrista sp ), Dysmicoccus sp , Scale insects (Aspidiotus destructor, Diaspis boisduvalii ), Thrips (Thrips florum, Franckliniella spp, Chaetanaphothrips signipennis ), Banana fruit Philippines, -
Processing of Coffee
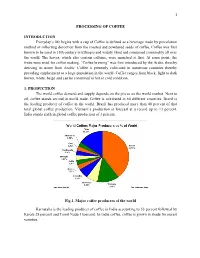
1 PROCESSING OF COFFEE INTRODUCTION Everyday’s life begins with a cup of Coffee is defined as a beverage made by percolation method or collecting decoction from the roasted and powdered seeds of coffee. Coffee was first known to be used in 15th century in Ethiopia and widely liked and consumed commodity all over the world. The leaves, which also contain caffeine, were munched at first. At some point, the fruits were used for coffee making. “Coffee brewing” was first introduced by the Arabs, thereby deriving its name from Arabic. Coffee is presently cultivated in numerous countries thereby providing employment to a large population in the world. Coffee ranges from black, light to dark brown, white, beige and can be consumed in hot or cold condition. 1. PRODUCTION The world coffee demand and supply depends on the prices on the world market. Next to oil, coffee stands second in world trade. Coffee is cultivated in 60 different countries. Brazil is the leading producer of coffee in the world. Brazil has produced more than 40 percent of that total global coffee production. Vietnam’s production is forecast at a record up to 13 percent. India stands sixth in global coffee production of 3 percent. Fig.1. Major coffee producers of the world Karnataka is the leading producer of coffee in India accounting to 53 percent followed by Kerala 28 percent and Tamil Nadu 11percent. In India coffee, coffee is grown in shade for purest varieties. 2 2 Varieties: Among the nearly 60 varieties of coffee, Arabica, Robusta and Liberica are three major varieties grown worldwide. -

Ethnoentomological and Distributional Notes on Cerambycidae and Other Coleoptera of Guerrero and Puebla,Mexico
The Coleopterists Bulletin, 71(2): 301–314. 2017. ETHNOENTOMOLOGICAL AND DISTRIBUTIONAL NOTES ON CERAMBYCIDAE AND OTHER COLEOPTERA OF GUERRERO AND PUEBLA,MEXICO JONATHAN D. AMITH Research Affiliate, Department of Anthropology, Gettysburg College, Campus Box 2895, Gettysburg, PA 17325, U.S.A. and Research Associate, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, U.S.A. AND STEVEN W. LINGAFELTER Systematic Entomology Laboratory, Agriculture Research Service, United States Department of Agriculture, National Museum of Natural History, Smithsonian Institution,Washington, DC 20013-7012, U.S.A. Current address: 8920 South Bryerly Ct., Hereford, AZ 85615, U.S.A. ABSTRACT This article presents both ethnoentomological notes on Nahuatl and Mixtec language terms as they are applied to Cerambycidae (Coleoptera) and distributional records for species collected during three projects carried out in the states of Guerrero and Puebla, Mexico. Some comparative data from other Mesoamerican and Native American languages are discussed. Indigenous common names are mapped onto current taxonomic nomenclature, and an analysis is offered of the logical basis for Indigenous classification: the exclusion of some cerambycids and the inclusion of other beetles in the nominal native “cerambycid” category. New state distributional records for the Cerambycidae collected in this study are offered for Guerrero: Bebelis picta Pascoe, Callipogon senex Dupont, Neocompsa macrotricha Martins, Olenosus ser- rimanus Bates, Ornithia mexicana zapotensis Tippmann, Stenygra histrio Audinet-Serville, Strongylaspis championi Bates, Lissonotus flavocinctus puncticollis Bates, and Nothopleurus lobigenis Bates; and Puebla: Juiaparus mexicanus (Thomson), Ptychodes guttulatus Dillon and Dillon, and Steirastoma senex White. Key Words: linguistics, etymology, Nahuatl, Mixtec, longhorned beetle, wood-borer DOI.org/10.1649/0010-065X-71.2.301 The present article emerges from two language shapes. -

Morning Coffee in the Clouds
MORNING COFFEE IN THE CLOUDS Arrival daily from 7 am to 9 am Minimum Spend AED 100 per person Tables upon availability SIGNATURE COFFEE Kopi Luwak 120 The world’s most premium coffee - 100% Wild Kopi Luwak from Indonesia. Smooth, balanced, rich flavour and complex aroma, nutty with notes of caramel. You can enjoy it on classic Italian way as an espresso, cappuccino or with warm milk on the side. Kopi Luwak Martini 140 Elegant mocktail made with our signature infusion of Kopi Luwak beans, Vanilla sticks from Madagascar, premium Brazilian oranges and sugar cane syrup. Best way to start your day on the top of the World. Kopi Luwak Gold Cappuccino 160 Our signature Gold Cappuccino with 24-karat gold flakes. Magnificent coffee drink in the World’s highest Restaurant & Lounge will be one of the most unique experiences of your life. Mini Dessert Platter 80 Cheesecake, vanilla scone, chocolate scone, madeleines, pate de fruit. All prices are in AED and are inclusive of %10 Municipality Fee and %10 Service Charge. VAT of %5 has been added to the net value of the mentioned prices. REFRESHMENTS Red Sand 45 Red of Arabia tea with the hint of ginger blended with yuzu and Caribbean orange. Afternoon Delight 45 Happy hour tea blended with fresh fennel, peach, fresh pineapple and passion fruit caviar. Miraculous Mandarin 45 Miraculous Mandarin iced tea with spiced ginger and pear Tropical Smile 45 Fresh passion fruit and mango layered with coconut flavored cream. High Berry 45 Pink Flamingo tea blended with black currant, cranberry and elderflowers. -

Color Chart.Pdf
® Finishing Products Division of RPM Wood Finishes Group Inc. Color Chart The Original Touch Up Company™ Made in the USA Color Chart ® Finishing Products Division of RPM Wood Finishes Group, Inc. Index Aerosols 1-5 Ultra® Classic Toner & Tone Finish Toner 1-3 Colored Lacquer Enamel 3-5 Shadow Toner 5 Touch-Up Markers/Pencils 5-15 Ultra® Mark Markers 5-9 3 in 1 Repair Stick 9 Pro-Mark® Markers 9-10 Quik-Tip™ Markers 10-11 Background Marker Touch-Up & Background Marker Glaze Hang-Up 11-13 Artisan Glaze Markers 13 Vinyl Marker Glaze Hang-Up 14 Brush Tip Graining Markers 14 Accent Pencils 15 Blend-Its 15 Fillers 15-29 Quick Fill® Burn-In Sticks 15-16 Edging/Low Heat Sticks 16 E-Z Flow™ Burn-In Sticks 16-17 PlaneStick® Burn-In Sticks 17-18 Fil-Stik® Putty Sticks 18-25 Hard Fill & Hard Fill Plus 25-27 PermaFill™ 27 Epoxy Putty Sticks 27-28 Patchal® Puttys 28-29 Knot Filler 29 Fil-O-Wood™ Wood Putty Tubes 29 Color Replacement 30-31 Blendal® Sticks 30 Sand Thru Sticks 30-31 Blendal® Powder Stains 31 Bronzing Powders 31 Dye Stains 32 Ultra® Penetrating & Architectural Ultra® Penetrating Stain 32 Dye Concentrate 32 Pigmented Stains 32-34 Wiping Wood™, Architectural Wiping Stain & Wiping Wood™ Stain Aerosols 32-33 Designer Series Stain, Designer Series Radiant Stain 33-34 Glazes 34 Finisher’s Glaze™ Glazing Stain & Aerosols 34 Break-A-Way™ Glaze & Aerosols 34 Leather Repair 35-37 E-Z Flow™ Leather Markers 35 Leather/Vinyl Markers 35 Leather/Vinyl Fil Sticks 35-36 Leather Repair Basecoat Aerosols 36 Leather Repair Toner Aerosols 36 Leather Repair Color Adjuster Aerosols 37 Touch Up Pigment 37 Leather Refinishing 37 Base Coat 37 NOTE: COLORS ARE APPROXIMATE REPRESENTATIONS OF ACTUAL COLORS USING MODERN PROCESS TECHNIQUES. -

The Effects of Climate Change on the Pests and Diseases of Coffee
y & W log ea to th a e im r l F o Journal of Climatology & Weather C f r e o c l a a s n Groenen, Climatol Weather Forecasting 2018, 6:3 t r i n u g o J Forecasting DOI: 10.4172/2332-2594.1000239 ISSN: 2332-2594 Review Article Open Access The Effects of Climate Change on the Pests and Diseases of Coffee Crops in Mesoamerica Danielle Groenen* Department of Earth, Ocean and Atmospheric Science and Center for Ocean–Atmospheric Prediction Studies, Florida State University, Florida, United States *Corresponding author: Danielle Groenen, Department of Earth, Ocean and Atmospheric Science and Center for Ocean–Atmospheric Prediction Studies, Florida State University, Florida, United States, Tel: 18506442525; E-mail: [email protected] Received Date: August 30, 2018; Accepted Date: September 21, 2018; Published Date: September 26, 2018 Copyright: © 2018 Groenen D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Coffee is an in-demand commodity that is being threatened by climate change. Increasing temperatures and rainfall variability are predicted in the region of Mexico and Central America (Mesoamerica). This region is plagued with pests and diseases that have already caused millions of dollars in damages and losses to the coffee industry. This paper examines three pests that negatively affect coffee plants: the coffee borer beetle, the black twig borer, and nematodes. In addition, this paper examines three diseases that can destroy coffee crops: bacterial blight, coffee berry disease, and coffee leaf rust.