Relationship Between the Na+/H' Antiporter and Na+/Substrate Symport in Bacillus Alcalophilus (Nonalkalophilic Mutant/Efflux/Vesicles) ARTHUR A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cystine–Glutamate Antiporter Xct Deficiency Suppresses Tumor Growth While Preserving Antitumor Immunity
Cystine–glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity Michael D. Arensmana, Xiaoran S. Yanga, Danielle M. Leahya, Lourdes Toral-Barzaa, Mary Mileskia, Edward C. Rosfjorda, Fang Wanga, Shibing Dengb, Jeremy S. Myersa, Robert T. Abrahamb, and Christina H. Enga,1 aOncology Research & Development, Pfizer, Pearl River, NY 10965; and bOncology Research & Development, Pfizer, San Diego, CA 92121 Edited by William G. Kaelin Jr., Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, and approved April 2, 2019 (received for review September 1, 2018) T cell-invigorating cancer immunotherapies have near-curative Thus, tumor cells may rely on xCT to fulfill the majority of their potential. However, their clinical benefit is currently limited, as cysteine and GSH needs by importing cystine. only a fraction of patients respond, suggesting that these regimens Inhibition of xCT has been investigated as a therapeutic may benefit from combination with tumor-targeting treatments. As strategy for cancer based on observations that elevated xCT ex- oncogenic progression is accompanied by alterations in metabolic pression on tumor cells correlates with poor prognosis (10–12) pathways, tumors often become heavily reliant on antioxidant and that inhibition of xCT in preclinical studies suppresses tumor machinery and may be susceptible to increases in oxidative stress. growth (10, 12–14). However, these studies relied heavily on the The cystine–glutamate antiporter xCT is frequently overexpressed in use of sulfasalazine, a clinical compound used for the treatment cancer and fuels the production of the antioxidant glutathione; thus, of rheumatoid arthritis, ulcerative colitis, and Crohn’s disease. -

Cellular Transport Notes About Cell Membranes
Cellular Transport Notes @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey About Cell Membranes • All cells have a cell membrane • Functions: – Controls what enters and exits the cell to maintain an internal balance called homeostasis TEM picture of a – Provides protection and real cell membrane. support for the cell @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 1 About Cell Membranes (continued) 1.Structure of cell membrane Lipid Bilayer -2 layers of phospholipids • Phosphate head is polar (water loving) Phospholipid • Fatty acid tails non-polar (water fearing) • Proteins embedded in membrane Lipid Bilayer @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey Polar heads Fluid Mosaic love water Model of the & dissolve. cell membrane Non-polar tails hide from water. Carbohydrate cell markers Proteins @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 2 About Cell Membranes (continued) • 4. Cell membranes have pores (holes) in it • Selectively permeable: Allows some molecules in and keeps other molecules out • The structure helps it be selective! Pores @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey Structure of the Cell Membrane Outside of cell Carbohydrate Proteins chains Lipid Bilayer Transport Protein Phospholipids Inside of cell (cytoplasm) @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 3 Types of Cellular Transport • Passive Transport celldoesn’tuseenergy 1. Diffusion 2. Facilitated Diffusion 3. Osmosis • Active Transport cell does use energy 1. -

Transport of Sugars
BI84CH32-Frommer ARI 29 April 2015 12:34 Transport of Sugars Li-Qing Chen,1,∗ Lily S. Cheung,1,∗ Liang Feng,3 Widmar Tanner,2 and Wolf B. Frommer1 1Department of Plant Biology, Carnegie Institution for Science, Stanford, California 94305; email: [email protected] 2Zellbiologie und Pflanzenbiochemie, Universitat¨ Regensburg, 93040 Regensburg, Germany 3Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, California 94305 Annu. Rev. Biochem. 2015. 84:865–94 Keywords First published online as a Review in Advance on glucose, sucrose, carrier, GLUT, SGLT, SWEET March 5, 2015 The Annual Review of Biochemistry is online at Abstract biochem.annualreviews.org Soluble sugars serve five main purposes in multicellular organisms: as sources This article’s doi: of carbon skeletons, osmolytes, signals, and transient energy storage and as 10.1146/annurev-biochem-060614-033904 transport molecules. Most sugars are derived from photosynthetic organ- Copyright c 2015 by Annual Reviews. isms, particularly plants. In multicellular organisms, some cells specialize All rights reserved in providing sugars to other cells (e.g., intestinal and liver cells in animals, ∗ These authors contributed equally to this review. photosynthetic cells in plants), whereas others depend completely on an ex- Annu. Rev. Biochem. 2015.84:865-894. Downloaded from www.annualreviews.org ternal supply (e.g., brain cells, roots and seeds). This cellular exchange of Access provided by b-on: Universidade de Lisboa (UL) on 09/05/16. For personal use only. sugars requires transport proteins to mediate uptake or release from cells or subcellular compartments. Thus, not surprisingly, sugar transport is criti- cal for plants, animals, and humans. -

Renal Membrane Transport Proteins and the Transporter Genes
Techno e lo n g Gowder, Gene Technology 2014, 3:1 e y G Gene Technology DOI; 10.4172/2329-6682.1000e109 ISSN: 2329-6682 Editorial Open Access Renal Membrane Transport Proteins and the Transporter Genes Sivakumar J T Gowder* Qassim University, College of Applied Medical Sciences, Buraidah, Kingdom of Saudi Arabia Kidney this way, high sodium diet favors urinary sodium concentration [9]. AQP2 has a role in hereditary and acquired diseases affecting urine- In humans, the kidneys are a pair of bean-shaped organs about concentrating mechanisms [10]. AQP2 regulates antidiuretic action 10 cm long and located on either side of the vertebral column. The of arginine vasopressin (AVP). The urinary excretion of this protein is kidneys constitute for less than 1% of the weight of the human body, considered to be an index of AVP signaling activity in the renal system. but they receive about 20% of blood pumped with each heartbeat. The Aquaporins are also considered as markers for chronic renal allograft renal artery transports blood to be filtered to the kidneys, and the renal dysfunction [11]. vein carries filtered blood away from the kidneys. Urine, the waste fluid formed within the kidney, exits the organ through a duct called the AQP4 ureter. The kidney is an organ of excretion, transport and metabolism. This gene encodes a member of the aquaporin family of intrinsic It is a complicated organ, comprising various cell types and having a membrane proteins. These proteins function as water-selective channels neatly designed three dimensional organization [1]. Due to structural in the plasma membrane. -

Passive and Active Transport
Passive and Active Transport 1. Thermodynamics of transport 2. Passive-mediated transport 3. Active transport neuron, membrane potential, ion transport Membranes • Provide barrier function – Extracellular – Organelles • Barrier can be overcome by „transport proteins“ – To mediate transmembrane movements of ions, Na+, K+ – Nutrients, glucose, amino acids etc. – Water (aquaporins) 1) Thermodynamics of Transport • Aout <-> Ain (ressembles a chemical equilibration) o‘ • GA - G A = RT ln [A] • ∆GA = GA(in) - GA(out) = RT ln ([A]in/[A]out) • GA: chemical potential of A o‘ • G A: chemical potential of standard state of A • If membrane has a potential, i.e., plasma membrane: -100mV (inside negative) then GA is termed the electrochemical potential of A Two types of transport across a membrane: o Nonmediated transport occurs by passive diffusion, i.e., O2, CO2 driven by chemical potential gradient, i.e. cannot occur against a concentration gradient o Mediated transport occurs by dedicated transport proteins 1. Passive-mediated transport/facilitated diffusion: [high] -> [low] 2. Active transport: [low] -> [high] May require energy in form of ATP or in form of a membrane potential 2) Passive-mediated transport Substances that are too large or too polar to diffuse across the bilayer must be transported by proteins: carriers, permeases, channels and transporters A) Ionophores B) Porins C) Ion Channels D) Aquaporins E) Transport Proteins A) Ionophores Organic molecules of divers types, often of bacterial origin => Increase the permeability of a target membrane for ions, frequently antibiotic, result in collapse of target membrane potential by ion equilibration 1. Carrier Ionophore, make ion soluble in membrane, i.e. valinomycin, 104 K+/sec 2. -

The Electrochemical Gradient of Protons and Its Relationship to Active Transport in Escherichia Coli Membrane Vesicles
Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 1892-1896, June 1976 Biochemistry The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles (flow dialysis/membrane potential/energy transduction/lipophilic cations/weak acids) SOFIA RAMOS, SHIMON SCHULDINER*, AND H. RONALD KABACK The Roche Institute of Molecular Biology, Nutley, New Jersey 07110 Communicated by B. L. Horecker, March 17, 1976 ABSTRACT Membrane vesicles isolated from E. coli gen- presence of valinomycin), a respiration-dependent membrane erate a trans-membrane proton gradient of 2 pH units under potential (AI, interior negative) of approximately -75 mV in appropriate conditions when assayed by flow dialysis. Using E. coli membrane vesicles has been documented (6, 13, 14). the distribution of weak acids to measure the proton gradient (ApH) and the distribution of the lipophilic cation triphenyl- Moreover it has been shown that the potential causes the ap- methylphosphonium to measure the electrical potential across pearance of high affinity binding sites for dansyl- and azido- the membrane (AI), the vesicles are shown to generate an phenylgalactosides on the outer surface of the membrane (4, electrochemical proton gradient (AiH+) of approximately -180 15) and that the potential is partially dissipated as a result of mV at pH 5.5 in the presence of ascorbate and phenazine lactose accumulation (6). Although these findings provide ev- methosulfate, the major component of which is a ApH of about idence for the chemiosmotic hypothesis, it has also been dem- -110 mV. As external pH is increased, ApH decreases, reaching o at pH 7.5 and above, while AI remains at about -75 mV and onstrated (6, 16) that vesicles are able to accumulate lactose and internal pH remains at pH 7.5. -

Amino Acid Transporters As Tetraspanin TM4SF5 Binding Partners Jae Woo Jung1,Jieonkim2,Eunmikim2 and Jung Weon Lee 1,2
Jung et al. Experimental & Molecular Medicine (2020) 52:7–14 https://doi.org/10.1038/s12276-019-0363-7 Experimental & Molecular Medicine REVIEW ARTICLE Open Access Amino acid transporters as tetraspanin TM4SF5 binding partners Jae Woo Jung1,JiEonKim2,EunmiKim2 and Jung Weon Lee 1,2 Abstract Transmembrane 4 L6 family member 5 (TM4SF5) is a tetraspanin that has four transmembrane domains and can be N- glycosylated and palmitoylated. These posttranslational modifications of TM4SF5 enable homophilic or heterophilic binding to diverse membrane proteins and receptors, including growth factor receptors, integrins, and tetraspanins. As a member of the tetraspanin family, TM4SF5 promotes protein-protein complexes for the spatiotemporal regulation of the expression, stability, binding, and signaling activity of its binding partners. Chronic diseases such as liver diseases involve bidirectional communication between extracellular and intracellular spaces, resulting in immune-related metabolic effects during the development of pathological phenotypes. It has recently been shown that, during the development of fibrosis and cancer, TM4SF5 forms protein-protein complexes with amino acid transporters, which can lead to the regulation of cystine uptake from the extracellular space to the cytosol and arginine export from the lysosomal lumen to the cytosol. Furthermore, using proteomic analyses, we found that diverse amino acid transporters were precipitated with TM4SF5, although these binding partners need to be confirmed by other approaches and in functionally relevant studies. This review discusses the scope of the pathological relevance of TM4SF5 and its binding to certain amino acid transporters. 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; Introduction on the plasma membrane and the mitochondria, lyso- Importing and exporting biological matter in and out of some, and other intracellular organelles. -

Membrane Transport Quiz
Membrane Transport Quiz 1. Which of the following is an example of extracellular fluid? a. Cytosol b. Plasma c. Interstitial Fluid d. Both b and c 2. Which of the following correctly describes passive transport? a. the cell uses ATP in passive transport b. most pumps are examples of passive transport c. diffusion is an example of passive transport d. exocytosis is an example of passive transport 3. Simple diffusion occurs ______________. a. with transporters in the cell membrane b. directly across the cell membrane c. through exocytosis d. through endocytosis 4. Which of the following is an example of active transport? a. Filtration b. Osmosis c. Endocytosis d. Exocytosis e. Both c and d 5. Which type of active transport uses ATP directly? a. Primary Active Transport b. Secondary Active Transport c. Both a and b 6. Which of the following is an example of receptor mediated endocytosis? a. Phagocytosis b. Primary Active Transport c. Exocytosis d. ALL are For use with TCC iTunes University Membrane Transport Lecture. 1 Developed by: Martha Kutter 2009 for the Learning Commons at Tallahassee Community College. 7. A transporter that moves one type of particle in one direction is _______________. a. Uniporter b. Symporter c. Antiporter 8. A transporter the moves two different particles in two different directions is ________. a. Endocytosis b. Exocytosis c. Uniporter d. Symporter e. Antiporter 9. Which of the following is an example of a primary active transporter? a. Na+/Ca2+ transporter on cardiac contractile cells b. Na+ channels on neurons c. Na+/K+ ATPase on all cells d. -

Specific Copb Transporter: Revising P1B-Type Atpase Classification
+ Cu -specific CopB transporter: Revising P1B-type ATPase classification Rahul Purohita,b, Matthew O. Rossa,b, Sharon Bateluc, April Kusowskic,d, Timothy L. Stemmlerc,d, Brian M. Hoffmana,b, and Amy C. Rosenzweiga,b,1 aDepartment of Molecular Biosciences, Northwestern University, Evanston, IL 60208; bDepartment of Chemistry, Northwestern University, Evanston, IL 60208; cDepartment of Pharmaceutical Sciences, Wayne State University, Detroit, MI 48201; and dSchool of Medicine, Wayne State University, Detroit, MI 48201 Contributed by Amy C. Rosenzweig, January 12, 2018 (sent for review December 14, 2017; reviewed by Megan M. McEvoy and Gabriele Meloni) The copper-transporting P1B-ATPases, which play a key role in cellu- metal specificities of the P1B-5 (PCP motif), P1B-6 (SCA motif), lar copper homeostasis, have been divided traditionally into two and P1B-7-ATPases (CSC motif) remain unclear, although some 2+ 2+ subfamilies, the P1B-1-ATPases or CopAs and the P1B-3-ATPases or evidence links the P1B-5-ATPases to Ni and Fe (20, 21). The + CopBs. CopAs selectively export Cu whereas previous studies and remaining two groups are the copper transporters. The P1B-1- + bioinformatic analyses have suggested that CopBs are specific for ATPases, which include ATP7A and ATP7B, transport Cu 2+ 2+ Cu export. Biochemical and spectroscopic characterization of (9, 22), whereas the P1B-3-ATPases are proposed to transport Cu Sphaerobacter thermophilus CopB (StCopB) show that, while it does (23, 24). These two subfamilies differ from one another in several 2+ bind Cu , the binding site is not the prototypical P1B-ATPase trans- ways. First, the TM helix 4 motif is CPC in the P1B-1-ATPases and membrane site and does not involve sulfur coordination as proposed CPH in the P1B-3-ATPases. -

Biology Passive & Active Transport April 30, 2020
High School Science Virtual Learning Biology Passive & Active Transport April 30, 2020 High School General Biology Lesson: Passive & Active Transport Objective/Learning Target: Students will understand how passive and active transports work. Bell Ringer Activity 1. If someone is being active what does that mean? 2. If someone is being passive what does that mean? Bell Ringer Answers 1. If someone is being active that means they are marked by energetic activity. 2. If someone is being passive they are accepting what happens to others without an active response. Keep these definitions in mind as we discuss the differences between what active and passive transport are in biology. Let’s Get Started! Lesson Activity: Directions: 1. Watch this video. 2. Create a Venn Diagram like the one you see here ---> 3. Compare and contrast Active and Passive Transport by the information you learn from the video. Lesson Questions Answers Venn Diagram Examples: Practice Questions 1. What is passive transport? 2. What is active transport? 3. What is the difference between diffusion and osmosis? 4. What is the difference between endocytosis and exocytosis? 5. What is the differences between facilitated diffusion and active transport by a protein pump? Answers to Practice Questions 1. Passive transport is the movement of materials across the cell membrane without using cellular energy. 2. Active transport is the movement of materials against a concentration difference; it requires energy. 3. In diffusion, both solvent and solute particles are free to move; however, in osmosis only water molecules cross the semipermeable membrane. Answers to Practice Questions Continued 4. -
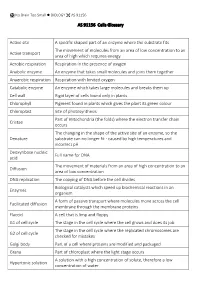
AS 91156 Cells Glossary Active Site a Specific Shaped Part of an Enzyme Where the Substrate Fits Active Transport the Movement
No Brain Too Small BIOLOGY AS 91156 AS 91156 Cells Glossary Active site A specific shaped part of an enzyme where the substrate fits The movement of molecules from an area of low concentration to an Active transport area of high which requires energy Aerobic respiration Respiration in the presence of oxygen Anabolic enzyme An enzyme that takes small molecules and joins them together Anaerobic respiration Respiration with limited oxygen Catabolic enzyme An enzyme which takes large molecules and breaks them up Cell wall Rigid layer of cells found only in plants Chlorophyll Pigment found in plants which gives the plant its green colour Chloroplast Site of photosynthesis Part of mitochondria (the folds) where the electron transfer chain Cristae occurs The changing in the shape of the active site of an enzyme, so the Denature substrate can no longer fit - caused by high temperatures and incorrect pH Deoxyribose nucleic Full name for DNA acid The movement of materials from an area of high concentration to an Diffusion area of low concentration DNA replication The copying of DNA before the cell divides Biological catalysts which speed up biochemical reactions in an Enzymes organism A form of passive transport where molecules move across the cell Facilitated diffusion membrane through the membrane proteins Flaccid A cell that is limp and floppy G1 of cell cycle The stage in the cell cycle where the cell grows and does its job The stage in the cell cycle where the replicated chromosomes are G2 of cell cycle checked for mistakes Golgi body Part -

Plasma Membrane Sandwich Model Unit Membrane
Plasma Membrane Sandwich Model Unit Membrane FLUID MOSAIC MODEL FLUID- because individual phospholipids and proteins can move side-to-side within the layer, like it’s a liquid. MOSAIC- because of the pattern produced by the scattered protein molecules when the membrane is viewed from above. 5 Functions • Protection and support – Maintains cell shape and size Solubility • Materials that are soluble in lipids can pass through the cell membrane easily 7 Selective Permeablility • Small molecules Ions, hydrophilic and larger molecules larger than hydrophobic water, and large molecules move molecules such as through easily. proteins do not move through the • e.g. O2, CO2, H2O membrane on their own. DIFFUSION Diffusion is a PASSIVE process which means no energy is used to make the molecules move, they have a natural KINETIC ENERGY 9 Passive Transport Simple Diffusion ❖ Doesn’t require energy ❖ Moves high to low concentration ❖ Example: Oxygen or water diffusing into a cell and carbon dioxide diffusing out. 10 Diffusion through a Membrane Cell membrane Solute moves DOWN concentration gradient (HIGH to LOW) 11 Osmosis • Diffusion of water Diffusion across a membrane across a membrane • Moves from HIGH water potential (low Semipermeable solute) to LOW water membrane potential (high solute) 12 Diffusion of H2O Across A Membrane High H O potential 2 Low H2O potential Low solute concentration High solute concentration 13 Passive Transport Facilitated diffusion ❖Doesn’t require energy ❖Uses transport proteins to move high to low concentration Examples: Glucose or amino acids moving from blood into a cell. 14 Types of Transport Proteins • Channel proteins are embedded in the cell membrane & have a pore for materials to cross • Carrier proteins can change shape to move material from one side of the membrane to the other 15 Facilitated Diffusion Molecules will randomly move through the pores in Channel Proteins.