Publication List Prof. Dr. Norbert Trautmann (Aug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Production of Radionuclides
CHAPTER 15 Production of Radionuclides Contents 15.1. General considerations 389 15.2. Irradiation yields 390 15.3. Second-order reactions 393 15.4. Target considerations 397 15.4.1. Physical properties 397 15.4.2. Chemical properties 398 15.5. Product specifications 399 15.5.1. Radiochemical processing 399 15.5.2. Specific activity 400 15.5.3. Labeling 400 15.5.4. Radiochemical purity 401 15.6. Recoil separations 402 15.6.1. Target recoil products 402 15.6.2. Hot atom reactions 403 15.6.3. The Szilard-Chalmers process 404 15.7. Fast radiochemical separations 406 15.7.1. Production of 11C labeled compounds 407 15.7.2. Auto-batch procedures 407 15.7.3. On-line procedures: gas-phase separation 409 15.7.4. On-line procedures: solvent extraction 412 15.7.5. Mass separator procedures 412 15.8. Exercises 412 15.9. Literature 414 This chapter discusses production of radionuclides for beneficial use in science, medicine and technology. The nuclear fundamentals for the production processes have been given in Chapters 11 to 14. The formation of radionuclides is discussed in several Chapters: e.g. cosmogenic reactions leading to the formation of short-lived radionuclides in nature (Ch. 5 and 10); thermonuclear reactions leading to the formation of long-lived radioactivity in the universe (Ch. 17); the synthesis of trans-uranium elements (Ch. 16 and 19-21). The production and isolation of separated fission products is treated separately (Ch. 19-21). This chapter discusses aspects of fundamental importance to the production of radionuclides by a variety of methods. -

Experimental Aspects of Geoneutrino Detection: Status and Perspectives
Experimental Aspects of Geoneutrino Detection: Status and Perspectives O. Smirnov,1 1JINR, Joint Institute for Nuclear Research, Dubna, Russian Federation October 22, 2019 Abstract Neutrino geophysics, the study of the Earth's interior by measuring the fluxes of geologically produced neutrino at its surface, is a new interdisciplinary field of science, rapidly developing as a synergy between geology, geophysics and particle physics. Geoneutrinos, antineutrinos from long- lived natural isotopes responsible for the radiogenic heat flux, provide valuable information for the chemical composition models of the Earth. The calculations of the expected geoneutrino signal are discussed, together with experimental aspects of geoneutrino detection, including the description of possible backgrounds and methods for their suppression. At present, only two detectors, Borexino and KamLAND, have reached sensitivity to the geoneutrino. The experiments accumulated a set of ∼190 geoneutrino events and continue the data acquisition. The detailed description of the experiments, their results on geoneutrino detection, and impact on geophysics are presented. The start of operation of other detectors sensitive to geoneutrinos is planned for the near future: the SNO+ detector is being filled with liquid scintillator, and the biggest ever 20 kt JUNO detector is under construction. A review of the physics potential of these experiments with respect to the geoneutrino studies, along with other proposals, is presented. New ideas and methods for geoneutrino detection are reviewed. Contents 1 Introduction 2 2 Geoneutrinos and the Earth's heat 4 2.1 Long-lived radiogenic elements . 4 2.2 Radiogenic heat and geoneutrino luminosity of the Earth . 9 arXiv:1910.09321v1 [physics.geo-ph] 21 Oct 2019 3 Geoneutrino flux calculation 11 3.1 Neutrino oscillations . -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title RADIOCHEMICAL AND SPECTROMETER STUDIES OF SEVERAL NEUTRON-DEFICIENT ZIRCONIUM ISOTOPES AND THEIR DECAY PRODUCTS Permalink https://escholarship.org/uc/item/5ck8q7xw Authors Hyde, Earl K. O'Kelley, Grover D. Publication Date 1950-12-28 eScholarship.org Powered by the California Digital Library University of California UCRL- .Jt)AJ,-.64.. _.; >-w .J w ... ~ ,• 0:: w m I . ;•· <( z 0:: 0 LL ..J u<( LL 0 >- 1-- TWO-WEEK LOAN COPY (/) This is a Library Circulating Copy 0:: which may be borrowed for two weeks. w For a personal retention copy, call > Tech. Info. Diuision, Ext. 5545 z '.-o:-. :J ~~ RADIATION LABORATORY DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any · information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. -

Technische Universität München Physik-Department Study of The
Technische Universität München Physik-Department Study of the biokinetics of zirconium isotopes in humans and its relevance to internal dosimetry Matthias Greiter Vollständiger Abdruck der von der Fakultät für Physik der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. J. L. van Hemmen Prüfer der Dissertation: 1. Hon.-Prof. Dr. H.G. Paretzke 2. Univ.-Prof. Dr. F. von Feilitzsch Die Dissertation wurde am 27.09.2007 bei der Technischen Universität München eingereicht und durch die Fakultät für Physik am 22.04.2008 angenommen. i Author contact information: Matthias Greiter Institute of Radiation Protection Helmholtz Zentrum München German Research Center for Environmental Health (GmbH) Ingolstaedter Landstrasse 1 85764 Neuherberg Germany E-mail: [email protected] ii Table of contents Abstract ...................................................................................................................................... 1 List of acronyms, symbols and abbreviations ............................................................................ 2 1 Aim of the study......................................................................................................... 3 1.1 Natural occurrence of zirconium compounds ............................................................ 3 1.2 Applications of stable zirconium................................................................................ 5 1.3 Applications -

Development of a National Neutron Database for Nuclear Technology
- 7 - XA9744992 DEVELOPMENT OF A NATIONAL NEUTRON DATABASE FOR NUCLEAR TECHNOLOGY A. V. Ignatyuk, V.N. Kononov, B.D. Kuzminov, V.N. Manokhin M.N. Nikolaev, B.I. Furzov State Research Centre of the Russian Federation Institute of Physics and Power Engineering, Obninsk Abstract This paper describes the stages of a many years activities at the IPPE consisting of the measurement, theoretical description and evaluation of neutron data, and of the establishment of a national data bank of neutron data for nuclear technology. A list of libraries which are stored at the Nuclear Data Centre is given. Introduction Currently, nuclear technology has found wide applications in a number of fields in science and technology consisting of: - nuclear energy (nuclear fission reactors, nuclear fission reactors, radioisotopic sources) - nuclear transmutation (production of radioisotopes for medical and other applications, burn-up of long-lived radio nuclides, radiation alloying of semiconducting materials, etc..) - nuclear instrumentation in industry (activation analysis, nuclear micro-analysis, control of fissile materials, etc..) - scientific investigations (nuclear physics, nuclear astrophysics, medicine, radiobiology, agro-radiology, ecology, etc.). In the sphere of nuclear technology, a number of processes use more than 500 stable and radioactive nuclides in a broad energy range. To ascertain the proper functioning of nuclear installations which have various functions and applications in industrial projects and scientific investigations, there is a need for nuclear data that characterize the properties of nuclear reactions. The reliability of radiation and nuclear safety, the ecological acceptance, and the economical planning of nuclear installations, all depend on the completeness, quality and accuracy of nuclear data. -
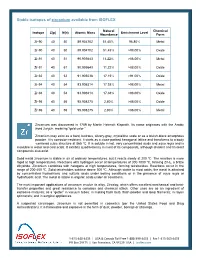
Stable Isotopes of Zirconium Available from ISOFLEX
Stable isotopes of zirconium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Zr-90 40 50 89.904702 51.45% 96.80% Metal Zr-90 40 50 89.904702 51.45% >98.00% Oxide Zr-91 40 51 90.905643 11.22% >88.00% Metal Zr-91 40 51 90.905643 11.22% >88.00% Oxide Zr-92 40 52 91.905038 17.15% >91.00% Oxide Zr-94 40 54 93.906314 17.38% >98.00% Metal Zr-94 40 54 93.906314 17.38% >98.00% Oxide Zr-96 40 56 95.908275 2.80% >86.00% Oxide Zr-96 40 56 95.908275 2.80% >86.00% Metal Zirconium was discovered in 1789 by Martin Heinrich Klaproth. Its name originates with the Arabic word zargun, meaning “gold color.” Zirconium may exist as a hard, lustrous, silvery-gray, crystalline scale or as a bluish-black amorphous powder. It is corrosion-resistant. It starts as a close-packed hexagonal lattice and transforms to a body -centered cubic structure at 865 ºC. It is soluble in hot, very concentrated acids and aqua regia and is insoluble in water and cold acids. It exhibits quadrivalency in most of its compounds, although divalent and trivalent compounds also exist. Solid metal zirconium is stable in air at ordinary temperatures, but it reacts slowly at 200 ºC. The reaction is more rapid at high temperatures. Reactions with hydrogen occur at temperatures of 300-1000 ºC, forming ZrH2, a brittle dihydride. Zirconium combines with halogens at high temperatures, forming tetrahalides. -

Tompbnents of Fission and 'Natural Radioactivity Found in Water Are
DOCUMENT RESUME,, A ED '170 1110 SE027 bill. 0 AUTHOR Thatcher, L.L. ;And Others 1 TITLE Techniques of Water-Resources Investigations of the United. States' Geblogical.Survey. Book 5, Laboratory, Analysis. Chapter- A5, Methodscr Dete-rmination of - Radioactive Substances in Water and Fluvial Sediments. INSTITUTION -Geological Survey (Dept,: .of Interior) ,Reston, PUB DATE 77 NO Tr 95p. ;For related document, see ED 131 218 AVAILABLE FRQM Super.intendent of Documents, U.S. Government Printing Off /ce , Washington, D.C. -20402(Stock Number 024- 0.01- 02928- 6:, No price quoted) */ FDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS *ChemiCal Analysis; *Geolo'gy; *LaboratorTechniques; PhYsic; Pollution; Retource Materials; S.c ence Equipment; Water Pollution control IDENTIFIERS *RadioactiveVs- Substances; *Sediments; Water Quality ABSTkACT Analytical methods for determining important , tompbnents of fission and 'natural radioactivity found in water are reportedThe discussion of each method includes conditions for application of the method, a summary of the method, interferences, required apparatus, Procedures, calculations and estimation of precision. Isotopes considered are cesium-137, strontium-90, lead-210, radium-226. and 228, tritium, and carbon-714. `When two methods are in use for an isotope,- both methods are reported. Techniques for the collection and preservation of water samples to be anallze?d for radioactivity are also discuSsed.(CS) v. *********************************************************************** 4 Re. productions supplied -

Technology Reference Guide for Radioactively Contaminated Media (Guide) As a Reference for Technologies That Can Effectively Treat Radioactively Contaminated Sites
DISCLAIMER This Technology Guide, developed by USEPA, is meant to be a summary of information available for technologies demonstrated to be effective for treatment of radioactively contaminated media. Inclusion of technologies in this Guide should not be viewed as an endorsement ofeither the technology or the vendor by USEPA. Similarly, exclusion of any technology should not be viewed as not being endorsed by USEPA; it merely means that the information related to that technology was not so readily available during the development of this Guide. Also, the technology-specific performance and cost data presented in this document are somewhat subjective as they are from a limited number of demonstration projects and based on professional judgment. In addition, all images used in this document are from public domain or have been used with permission. FORWARD The Technology Reference Guidance for Radioactively Contaminated Media (Guide) is intended to aid in the selection of treatment technologies for remediation of radioactively contaminated media. The Guide is designed to help site managers, Remedial Program Managers (RPM), On-Scene Coordinators (OSC), their contractors and others to identify and understand technologies that are potentially useful in the remediation of radioactively contaminated media. This Guide is designed to give easy access to critical information on applied technologies that address radioactive contamination in solid and liquid media. The solid media includes soils, sediments, sludge and solid waste, but does not include buildings and structures. The liquid media includes groundwater, surface water, leachate and waste water. The Guide is an update of the 1996 document "Technology Screening Guide for Radioactively Contaminated Site," EPA-402-R-96-017. -

US7736610.Pdf
USOO773661 OB2 (12) United States Patent (10) Patent No.: US 7,736,610 B2 Meikrantz et al. (45) Date of Patent: *Jun. 15, 2010 (54) ACTINUM RADIOSOTOPE PRODUCTS OF 2003/O127395 A1 7/2003 Bond et al. ENHANCED PURITY 2003/0194364 A1 10, 2003 Bond et al. 2004/0052705 A1 3/2004 Tranter et al. (75) Inventors: David Herbert Meikrantz, Idaho Falls, 2004.0062695 A1 4/2004 Horwitz et al. ID (US); Terry Allen Todd, Aberdeen, 2004/0254419 A1 12/2004 Wang et al. ID (US); Troy Joseph Tranter, Idaho OTHER PUBLICATIONS Falls, ID (US); E. Philip Horwitz, Naperville, IL (US) Benedict, Glen E., “Improvements in Thorium-Uranium Separation in the Acid-Thorex Process.” ACS Symposium Series #117. Ameri can Chemical Society, Washington, D.C. 1980; p. 371. (73) Assignee: Battelle Energy Alliance, LLC, Idaho Dietz et al., “An improved extraction chromatographic resin for the Falls, ID (US) separation of uranium from acidic nitrate media.” Talanta 54:1173 1184 (2001). (*) Notice: Subject to any disclaimer, the term of this Grant, G. R. et al..."Heavy Element Separation for Thorium Ura patent is extended or adjusted under 35 nium-Plutonium Fuels.” ACS Symposium Series #117. American U.S.C. 154(b) by 871 days. Chemical Society, Washington, D.C. 1980; p. 351. Horwitz et al. “The Extraction of DBP and MBP from Actinides: This patent is Subject to a terminal dis Application to the Recovery of Actinides from TBP-Sodium Carbon claimer. ate Scrub Solutions.” American Chemical Society pp. 475-496 (1980). (21) Appl. No.: 11/278,522 Horwitz et al., “New Extraction Chromatographic Resins for the Recovery of TRUs and Lanthanides from Acidic Nitrate and Chloride Filed: Apr. -

Ac-225 Research Beginning to Produce Results
Managed by the U.S. Department of Energy’s Office of Nuclear Physics NIDC Newsletter - # 03 April 2013 Ac-225 Research Beginning To Produce Results Inside This Issue Since the discovery of radioactivity, there has been recognition that radiation can play a role in the treatment of disease. This was exemplified by the use • Ac-225 Research of radium sources to treat patients with tumors. When artificial radioisotopes were discovered, the potential was expanded to include elements that • IDPRA R&D compose molecules of biologic significance. One element, iodine, could be used in the atomic form, as iodide, when its affinity for thyroid tissue was • Upcoming Meetings discovered. After World War II, the use of radioiodine to treat thyroid cancer and hyperthyroidism was developed. • Dr. Alun G. Jones Obituary Many other isotopes have been explored, either for their natural affinity for tissue such as phosphate for bone or when formed into a molecule that has the right biologic behavior, e.g. iododeoxyuridine which incorporates into rapidly dividing tissue. At other times, physical means were used to direct the therapeutic isotope to its target as when colloids of phosphorous, gold or yttrium were used to either keep the radiation in a given space or to catch the isotope in the capillaries of the target. In some instances, the interest shifted from beta emitters to alpha emitters. This became more interesting as the targeting molecules became more sophisticated and the uptake by the target was reduced. Unlike beta emitters, alpha emitters are cytotoxic regardless of the dose rate of the therapy. And, Thorium test foil target used to measure cross sections for proton given their higher relative biologic effectiveness, they can be lethal at lower production of Ac-225. -

Theoretical Potential of the CANDU Core for Transmutation of PWR TRU Nuclides
Proceedings of the Reactor Physics Asia 2015 (RPHA15) Conference Jeju, Korea, Sept. 16-18, 2015 Theoretical Potential of the CANDU Core for Transmutation of PWR TRU Nuclides Jinqi Lyu, Donny Hartanto, Mohammad Abdul Motalab and Yonghee Kim Department of Nuclear and Quantum Engineering, KAIST 291 Daehak-ro, Yusong-gu, Daejeon 34141, Republic of Korea *Corresponding author: [email protected] 1. Introduction 2. Analysis Models and Methods Transuranic (TRU) nuclides generated in spent fuels In this work, the analysis is done only for a 2-D fuel from the Light Water Reactor (LWR) pose a big assembly lattice because the core characteristics can be challenge in nuclear waste management due to its well determined through lattice analyses in CANDU potential diversion, high radiological toxicity and long core. The lattice calculation is performed for a lifetime. One of the potential approaches for dealing CANFLEX fuel bundle, as illustrated in Fig. 1, which with spent nuclear fuel is to fully utilize (transmute) the can improve the operating and safety margin, as well as TRU nuclides in nuclear reactors as nuclear fuels. the economics of a CANDU reactor. There have been many studies about utilizing TRU nuclides in CANDU reactors since many design features of CANDU reactor make it uniquely suitable for transmuting TRU nuclides, such as simple fabrication and handling of active fuels, precise management of core reactivity, high neutron economy and so on [1]. The concept of MOX (mixed oxides) fuel, which mixes actinides with natural uranium, and IMF (inert matrix fuel), which mixes actinides with silicon carbide, were proposed previously. -

Active Actinium Naturally Scarce but Synthetically Accessible, Gauthier J.-P
in your element Active actinium Naturally scarce but synthetically accessible, Gauthier J.-P. Deblonde and Rebecca J. Abergel discuss element 89 and its emergence as a candidate radio-theranostic metal for cancer treatment. n 1899, during the aftermath of applications, the day-scale decay of the now Marie and Pierre Curie’s resounding easily accessible 225Ac makes it a seemingly Idiscovery of both polonium and radium, ideal candidate for destroying tumour cells. André-Louis Debierne — one of their Its final decay to the stable and non-toxic French research fellows — partially isolated 209Bi is also beneficial when compared with another new element. Ambiguities in competitors under clinical evaluation, namely Debierne’s purification procedure from 227Th,228 Th and 230U, all of which alpha-decay pitchblende residues almost cost him the to stable, but toxic, Pb isotopes. credit for the discovery of element 89, A rising challenge for chemists resides with Friedrich Oskar Giesel independently in the handling of 225Ac intermediates and managing to isolate the same isotope just potentially harmful recoiling daughters — three years later. After some discussion, 221Fr, 217At, and 213Po. The design of in vivo Debierne’s actinium (for the Greek aktinos, stable carriers able to scavenge both actinium meaning ray) was preferred to Giesel’s and its alpha cascade raises hope for highly emanium; although both names highlight its efficient cancer therapies with no side effects. active alpha-particle emanations. Various strategies are under development, The discovery of Ac was a continuation of among which the encapsulation within the work of the Curies, but never had the same nanoparticles seems most promising, impact as its freshly discovered radioactive whereby tumour delivery of 225Ac through a neighbour, Ra.