Spring 2004 Gems & Gemology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Political Thought of Harry Oppenheimer, Under the Editorship of Kalim Rajab
THE JOURNAL OF THE HELEN SUZMAN FOUNDATION | ISSUE 81 | DECEMBER 2017 BOOK REVIEW Gary Ralfe retired from active business life in 2006 after forty A Man Of Africa : The Political years of service firstly with Anglo American Corporation, and then Thought of Harry Oppenheimer with De Beers where he was Managing Director for eight Edited by Kalim Rajab years. In addition to My forty-year career with Anglo American and De Beers developed the HSF he chairs the Board of Governors under the benign shadow of Harry Oppenheimer (HFO). To generations of Michaelhouse, the of managers and staff in what used to be called the greater group HFO fund-raising arm of the Alexandra Education was more than a chairman and leader. He was a living icon. I was Committee, the fund- not the first or the last young man or woman to join Anglo American raising arm of Business Against Crime and the because it was a force for good in Southern Africa, the most important Beyond Foundation engine of economic growth, industrialisation, urbanization and a which does community work in rural areas. better life for all. I associated with the credo attributed to HFO’s father Sir Ernest Oppenheimer that “the purpose of large corporations such as Anglo American is to make profits for its shareholders, but to do so in such a way as to make a real and lasting contribution to the welfare of the communities where it operates”. It is surprising that seventeen years after HFO’s death there is still no biography. I know that the family wisely wanted time to elapse before commissioning one. -

The Diamond Cartel
THE DIAMOND CARTEL Although the United States is the principal consumer of the world's dia- mond supply,' the diamond industry is so cartelized as to be virtually imper- vious to pressure by American users of diamonds. Within the past ten years industrial diamonds have become indispensable to various high-speed, pre- cision manufacturing operations.2 Since distribution of ninety-five percent of the world's diamond production is channelized through a single marketing combine, competitive forces within the industry have been short circuited.a Therefore consumer resistance has been impracticable and American efforts to obtain a better bargaining position have so far been ineffective. The cartel is completely foreign-controlled and operates entirely outside the United States,4 thus obviating effective action under the antitrust laws and making 1. Leslie, Diamonds as Tools Speed up Victory, DoMEsTIc CoxriRcE, Aug. 1945, p, 28. Today, the United States uses approximately 90% of the world's production of Indus- trial diamonds. § 29 Complaint, DeBeers Consolidated Mines Ltd. v. United States, 325 U.S. 212 (1945) [hereinafter referred to as Complaint]. This figure includes all cate- gories of industrial diamonds--ballas, boart and carbonado. For a description of the types of industrial diamonds, see Diamonds for Victory, FOREIGN COMMERCE, Jan. 17, 1942, p. 4; Industrial Diamonds,.FORTUNE, June 1935, p. 22. Carbonado is rare and almost entirely confined to Brazil and will not enter into the discussion hereafter. Post-war production should produce the following ratios of diamond types: out of every 10,000 carats 250 will be carbonado, 1,900 ballas, 1,975 gemstones, and 5,875 boart. -

Country by Country Reporting
COUNTRY BY COUNTRY REPORTING PUBLICATION REPORT 2018 (REVISED) Anglo American is a leading global mining company We take a responsible approach to the management of taxes, As we strive to deliver attractive and sustainable returns to our with a world class portfolio of mining and processing supporting active and constructive engagement with our stakeholders shareholders, we are acutely aware of the potential value creation we operations and undeveloped resources. We provide to deliver long-term sustainable value. Our approach to tax is based can offer to our diverse range of stakeholders. Through our business on three key pillars: responsibility, compliance and transparency. activities – employing people, paying taxes to, and collecting taxes the metals and minerals to meet the growing consumer We are proud of our open and transparent approach to tax reporting. on behalf of, governments, and procuring from host communities – driven demands of the world’s developed and maturing In addition to our mandatory disclosure obligations, we are committed we make a significant and positive contribution to the jurisdictions in economies. And we do so in a way that not only to furthering our involvement in voluntary compliance initiatives, such which we operate. Beyond our direct mining activities, we create and generates sustainable returns for our shareholders, as the Tax Transparency Code (developed by the Board of Taxation in sustain jobs, build infrastructure, support education and help improve but also strives to make a real and lasting positive Australia), the Responsible Tax Principles (developed by the B Team), healthcare for employees and local communities. By re-imagining contribution to society. -

Directory of Organisations and Resources for People with Disabilities in South Africa
DISABILITY ALL SORTS A DIRECTORY OF ORGANISATIONS AND RESOURCES FOR PEOPLE WITH DISABILITIES IN SOUTH AFRICA University of South Africa CONTENTS FOREWORD ADVOCACY — ALL DISABILITIES ADVOCACY — DISABILITY-SPECIFIC ACCOMMODATION (SUGGESTIONS FOR WORK AND EDUCATION) AIRLINES THAT ACCOMMODATE WHEELCHAIRS ARTS ASSISTANCE AND THERAPY DOGS ASSISTIVE DEVICES FOR HIRE ASSISTIVE DEVICES FOR PURCHASE ASSISTIVE DEVICES — MAIL ORDER ASSISTIVE DEVICES — REPAIRS ASSISTIVE DEVICES — RESOURCE AND INFORMATION CENTRE BACK SUPPORT BOOKS, DISABILITY GUIDES AND INFORMATION RESOURCES BRAILLE AND AUDIO PRODUCTION BREATHING SUPPORT BUILDING OF RAMPS BURSARIES CAREGIVERS AND NURSES CAREGIVERS AND NURSES — EASTERN CAPE CAREGIVERS AND NURSES — FREE STATE CAREGIVERS AND NURSES — GAUTENG CAREGIVERS AND NURSES — KWAZULU-NATAL CAREGIVERS AND NURSES — LIMPOPO CAREGIVERS AND NURSES — MPUMALANGA CAREGIVERS AND NURSES — NORTHERN CAPE CAREGIVERS AND NURSES — NORTH WEST CAREGIVERS AND NURSES — WESTERN CAPE CHARITY/GIFT SHOPS COMMUNITY SERVICE ORGANISATIONS COMPENSATION FOR WORKPLACE INJURIES COMPLEMENTARY THERAPIES CONVERSION OF VEHICLES COUNSELLING CRÈCHES DAY CARE CENTRES — EASTERN CAPE DAY CARE CENTRES — FREE STATE 1 DAY CARE CENTRES — GAUTENG DAY CARE CENTRES — KWAZULU-NATAL DAY CARE CENTRES — LIMPOPO DAY CARE CENTRES — MPUMALANGA DAY CARE CENTRES — WESTERN CAPE DISABILITY EQUITY CONSULTANTS DISABILITY MAGAZINES AND NEWSLETTERS DISABILITY MANAGEMENT DISABILITY SENSITISATION PROJECTS DISABILITY STUDIES DRIVING SCHOOLS E-LEARNING END-OF-LIFE DETERMINATION ENTREPRENEURIAL -

The-Diamond-Empire-Transcript.Pdf
1 MEDIA EDUCATION F O U N D A T I O N 60 Masonic St. Northampton, MA 01060 | TEL 800.897.0089 | [email protected] | www.mediaed.org The Diamond Empire Transcript Part 1 begins on pg. 1 Part 2 begins on pg. 15 PART 1: INTRODUCTION NARRATOR: On a hill above Johannesburg is the private estate of one of the wealthiest families on earth. They’ve made their fortune by manipulating markets and fixing prices. Their money has come from ordinary people all over the world who imagine they’re investing in something rare and valuable. But this is a family which deals in illusion. It presides over one of the last great cartels, an extraordinarily successful business empire worth $60 billion. This is the Oppenheimer family and their trade is diamonds. NARRATOR: The richest diamond jewelry market in the world is in New York. It’s a $10.8 billion business. Around 47th Street, there are twenty-five thousand people who buy, cut, polish and sell diamonds, from the most glamorous jewelry to the cheapest mail-order. It’s a highly secretive and risky business. The Oppenheimers themselves declined to help in any way in the making of this film. Bill Goldberg is one of the few top dealers who will talk openly about what he does. BILL GOLDBERG: It’s fascinating, it’s amazing that a lot of men that are ten years younger than I am can’t wait to retire and I can’t wait to get here in the morning at eight o’clock, to produce these beautiful works, these beautiful pieces of art from what looks like a pebble on the beach. -

De Beers - Wikipedia
6/23/2020 De Beers - Wikipedia De Beers De Beers Group is an international corporation that specialises in diamond exploration, diamond mining, diamond retail, diamond De Beers Group trading and industrial diamond manufacturing sectors. The company is currently active in open-pit, large-scale alluvial, coastal and deep sea mining.[4] It operates in 35 countries and mining takes Industry Mining and trading place in Botswana, Namibia, South Africa, Canada and Australia. of diamonds From its inception in 1888 until the start of the 21st century, De Beers controlled 80% to 85% of rough diamond distribution and was Founded 1888 accused of being a monopoly.[5] Competition has since dismantled Founder Cecil Rhodes the complete monopoly, though the De Beers Group still sells London, United [6] Headquarters approximately 35% of the world's rough diamond production Kingdom through its global sightholder and auction sales businesses.[7] Area served Worldwide The company was founded in 1888 by British businessman Cecil Key people Mark Cutifani Rhodes, who was financed by the South African diamond magnate (Chairman) Alfred Beit and the London-based N M Rothschild & Sons bank.[8][9] Bruce Cleaver In 1926, Ernest Oppenheimer, a German immigrant to Britain and (CEO) later South Africa who had earlier founded mining company Anglo Products Diamonds American with American financier J.P. Morgan,[10] was elected to Diamond marketing the board of De Beers.[11] He built and consolidated the company's Services and promotion global monopoly over the diamond industry until his death in 1957. During this time, he was involved in a number of controversies, Revenue $6.08 billion including price fixing and trust behaviour, and was accused of not (2018)[1] releasing industrial diamonds for the U.S. -

PROSPECTUS US$1,500,000,000 Anglo American
PROSPECTUS US$1,500,000,000 Anglo American Capital plc US$850,000,000 3.625% Senior Notes due 2020 US$650,000,000 4.875% Senior Notes due 2025 Guaranteed by Anglo American plc This prospectus is being published by Anglo American Capital plc (the “Issuer”) in connection with Admission (as defined below) of its US$850 million of its 3.625% Senior Notes due 2020 (the “2020 Notes”) and US$650 million of its 4.875% Senior Notes due 2025 (the “2025 Notes” and, together with the 2020 Notes, the “Notes”) with such Notes to be guaranteed (the “Guarantees”) by Anglo American plc (the “Company”, “Guarantor” or “Anglo American” and, together with the Company’s subsidiaries, joint ventures and associates, “Anglo American Group”, the “Group”, “we”, “us” or “our”). Interest will be paid on the Notes semi-annually and in arrears on May 14 and November 14 of each year, commencing on November 14, 2015. The 2020 Notes and the 2025 Notes will mature on May 14, 2020 and May 14, 2025, respectively. The Issuer has the option to redeem all or a portion of the Notes at any time at the redemption prices set forth in this document. The Notes will be unsecured senior obligations of the Issuer and will rank equally with all of its other existing and future unsubordinated indebtedness. The Notes will be issued in fully registered form and only in denominations of US$200,000 and integral multiples of US$1,000 in excess thereof. For a more detailed description of the Notes, see “Description of the Notes and the Guarantees” beginning on page 141. -

The Heart of the Matter
THE HEART OF THE MATTER SIERRA LEONE, DIAMONDS & HUMAN SECURITY (COMPLETE REPORT) Ian Smillie Lansana Gberie Ralph Hazleton Partnership Africa Canada (PAC) is a coalition of Canadian and African organizations that work in partnership to promote sustainable human development policies that benefit African and Canadian societies. The Insights series seeks to deepen understanding of current issues affecting African development. The series is edited by Bernard Taylor. The Heart of the Matter: Sierra Leone, Diamonds and Human Security (Complete Report) Ian Smillie, Lansana Gberie, Ralph Hazleton ISBN 0-9686270-4-8 © Partnership Africa Canada, January 2000 Partnership Africa Canada 323 Chapel St., Ottawa, Ontario, Canada K1N 7Z2 [email protected] P.O. Box 60233, Addis Ababa, Ethiopia pac@ telecom.net.et ________________ The Authors Ian Smillie, an Ottawa-based consultant, has 30 years of international development experience, as manager, programmer, evaluator and writer. He was a founder of the Canadian NGO Inter Pares, and was Executive Director of CUSO from 1979 to 1983. His most recent publications include The Alms Bazaar: Altruism Under Fire; Non Profit Organizations and International Development (IT Publications, London, 1995) and Stakeholders: Government-NGO Partnerships for International Development (ed. With Henny Helmich, Earthscan, London, 1999). Since 1997 he has worked as an associate with the Thomas J. Watson Jr. Institute at Brown University on issues relating to humanitarianism and war. Ian Smillie started his international work in 1967 as a teacher in Koidu, the centre of Sierra Leone’s diamond mining area. Lansana Gberie is a doctoral student at the University of Toronto and research associate at the Laurier Centre for Military, Strategic and Disarmament Studies, Waterloo, Ontario. -
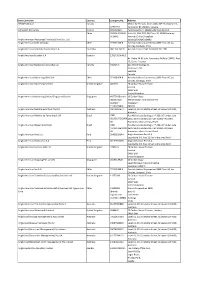
Data Controller Country Company No. Address 0912055 BC Ltd
Data Controller Country Company No. Address 0912055 BC Ltd Canada McCarthy Tetrault, Suite 2400, 745 Thurlow Street, C0912055 Vancouver BC V6E0C5, Canada AA Sakatti Mining Oy Finland FI24367683 Tuohiaavantie 2, 99600 Sodankylä Finland China 91310115MA1K Units 01, 02A, 07A, 08, Floor 32, 1198 Centurey 3Y1W42 Avenue, China (Shanghai) Anglo American [Resources] Trading (China) Co., Ltd. 91310115MA1K3Y1W42 Anglo American Chile Limitada. Chile 77.905.330-K Avenida Isidora Goyenechea 2800 Floor 46, Las Condes, Santiago, Chile Anglo American Colombia Exploration S.A Colombia 900.153.737-0 Avenida Carrera 9 #115-06/30º Ofi.1702 Anglo American Ecuador S.A. Ecuador 1792792940001 Av. Patria E4-69 y Av. Amazonas Edificio COFIEC, Piso 17, Quito, Ecuador Anglo American Exploration (Canada) Ltd Canada 332725-6 620-650 W Georgia St. Vancouver, BC V6B 4N8 Canada Anglo American Marketing Chile SpA Chile 76.908.604-8 Avenida Isidora Goyenechea 2800 Floor 46, Las Condes, Santiago, Chile Anglo American Marketing Limited United Kingdom 405724 20 Carlton House Terrace London SW1Y 5AN United Kingdom Anglo American Marketing Limited Singapore Branch Singapore 405724 (Branch 10 Collyer Quay registration #38-00 Ocean Financial Centre number: Singapore T11FC0199H) 049315 Anglo American Metallurgical Coal Pty Ltd Australia 93076059679 Level 11, 201 Charlotte Street, Brisbane Qld 4000, Australia Anglo American Minério de Ferro Brasil S/A Brazil CNPJ Rua Maria Luiza Santiago, nº 200, 16º andar, sala 02.359.572/0004-1601, Bairro Santa Lúcia, CEP 30360-740, Belo 30 Horizonte, Minas Gerais, Brazil Anglo American Níquel Brasil Ltda. Brazil CNPJ Rua Maria Luiza Santiago, nº 200, 16º andar, sala 42.184.226/0019-1601, Bairro Santa Lúcia, CEP 30360-740, Belo 69 Horizonte, Minas Gerais, Brazil Anglo American Peru S.A. -

A Review of Debswana‟S Performance Pre and Post the Acquisition of De Beers Shares by Anglo American Plc
A review of Debswana‟s performance pre and post the acquisition of De Beers shares by Anglo American plc. Khumo Mothulatshipi A research report submitted to the Faculty of Engineering and the Built Environment, University of the Witwatersrand, in partial fulfillment of the requirements for the degree of Master of Science in Engineering. Johannesburg, 2015 Declaration I declare that this research report is my own unaided work. Where work of other authors has been used, it has been duly acknowledged. It is being submitted for the Master of Science in Mining Engineering to the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination to any other university. ………………………………………………………………. Signature …………… day of ……………………year ………………………………….. i Abstract The minerals industry has in the last decade witnessed volatility, uncertainty, complexity and ambiguity (VUCA). The diamond industry has not been immune to these challenges. These challenges coincided with the decision of the diamond mining family in 2011 to opt out of De Beers after approximately a century of being in control by selling their 40 % stake to Anglo American plc (AA) which already had 45 % stake in De Beers. This transaction increased Anglo American plc‟s stake in De Beers to 85% thus joining venture with the Government of the Republic of Botswana (GRB) under an already existing entity called Debswana. Botswana has been hailed as a beacon of success and a model African state both politically and economically mainly due to its effective management of economic proceeds generated mainly through Debswana diamonds. Something that might be seen as a possible threat to this economic stability is the merger and acquisition (M&A) between the GRB and AA. -

Another Giant Mining Fortune Represented in the Pilgrims Society (History’S Biggest Pandora’S Box!)
ANOTHER GIANT MINING FORTUNE REPRESENTED IN THE PILGRIMS SOCIETY (HISTORY’S BIGGEST PANDORA’S BOX!) Presented October 2017 by Charles Savoie Refused coverage by Dave Janda, Greg Hunter, And dozens of “alt media” You Tube channels! Refused coverage by 24 Hour Gold, Silver Seek, And the rest of the “Ha-Ha” Parade! (Save this in your Downloads File; read in several sessions.) In April we reviewed details on James Ben Ali Haggin (1827- 1914, Episcopalian; Pilgrims Society member known as “the greatest mine owner on earth”) in “Silver Mining Kingpin Opposed Silver Money.” In August we reviewed the Guggenheim mining dynasty in “Vast Mining Fortune Represented in Pilgrims Society World Money Trust.” This item will be about the South Africa Oppenheimer mining fortune (diamonds, gold, platinum, uranium, titanium, zinc, copper, cobalt, coal, chemicals, manganese, rhodium, nickel, iron etc.) and there was a marriage in 1660 between the Guggenheims and Oppenheimers, back in the Old World. Not only that, but Jakob Oppenheimer ran a bank at Hanover, Germany, where the first Rothschild was an apprentice during 1757-1763! Samuel Oppenheimer (1630-1703) was military supplier to King Leopold I of Hungary, Croatia and Bohemia. The Guggenheims appear to have mostly died out and those still bearing that name being of lesser influence, the fortune shifting into Episcopalian hands (The Lawson-Johnstons, Episcopalians and Pilgrims Society members). I realize those releases were way longer than most metals investors are used to reading (539 pages together) but the background on Haggin, the Guggenheims and the Lawson-Johnstons is extensive, and in fact, I only provided a basic outline on both. -

Debswana Diamond Company and the Government of Botswana: an HIV/Aids Public-Private Partnership Programme
African Natural Resources Center African Development Bank Debswana Diamond Company and the Government of Botswana: an HIV/Aids public-private partnership programme A CASE STUDY Case study Botswana’s HIV/AIDS public-private partnership programme 2 table of contents Case study Botswana’s HIV/AIDS public-private partnership programme 3 Index 4 Preface 7 Introduction 7 Public-private partnerships 8 Health sector in Botswana 9 The HIV/AIDS epidemic in Botswana 11 Public policy and the strategic decision to collaborate 11 The Debswana Strategic Partnership 14 The collaboration and alignment with the government 17 Impact 20 Lessons learnt 22 The HIV/AIDS programme sustainability 22 Challenges 23 Policy considerations for other countries 23 Lessons for other countries 26 Bibliography Figures 9 Figure 1: Accumulated mineral revenues and public investment (real) 12 Figure 2: Country map showing locations of Debswana mines 14 Figure 3: Organisational chart of institutional arrangements 18 Figure 4: National ARV program 19 Figure 5: Productive time lost due to illness at all sites, 2002 - 2015 19 Figure 6: Ill health retirements from all operations, 2002 - 2014 20 Figure 7: Summary of deaths in service at all operations, 2002 - 2015 Case study Botswana’s HIV/AIDS public-private partnership programme 4 Preface The African Development Bank established the African Natural Resources Center as a non-lending entity aimed at building the capacity of member governments in the region to manage natural resources, whether renewable (fishery, forestry, land and water) or non-renewable (minerals, oil and gas). As part of its mandate, the center benchmarks experiences from other regions and supports African governments in performing their custodial obligations by collaborating with regional institutions, the private sector, civil society organizations and donors.