A Broad Filter Between Call Frequency and Peripheral Auditory Sensitivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -

Mammals – Columbia
Mammals – Columbia NWR Family Genus Species Common Name Soricidae vagrans Vagrant shrew Sorex (Shrews) merriami Merriam’s shrew Parastrellus hesperus Canyon bat Corynihinus townsendii Townsend’s big-eared bat Eptesicus fuscus Big brown bat Antrozous pallidus Pallid bat Euderma maculatum Spotted bat Lasionycteris noctivagans Silver-haired bat Vespertilionidae (Vesper bats) californicus California myotis ciliolabrum Western small-footed myotis evotis Long-eared myotis Myotis lucifugus Little brown myotis volans Long-legged myotis yumaensis Yuma myotis thysanodes Fringed myotis Lepus californicus Black-tailed jackrabbit Leporidae (Rabbits & hares) Sylvilagus nuttallii Nuttall’s cottontail Marmota flaviventris Yellow-bellied marmot Sciuridae (Squirrels) Urocitellus washingtoni Washington ground squirrel Castoridae (Beavers) Castor canadensis Beaver Geomidae (Pocket gophers) Thomomys talpoides Northern pocket gopher Perognathus parvus Great Basin pocket mouse Heteromyidae (Heteromyids) Dipodomys ordii Ord’s kangaroo rat Reithrodontomys megalotis Western harvest mouse Peromyscus maniculatus Deer mouse Onychomys leucogaster Northern grasshopper mouse Neotoma cinerea Bushy-tailed woodrat Cricetidae (Cricetids) montanus Montane vole Microtus pennsylvanicus Meadow vole Lemmiscus curtatus Sagebrush vole Ondatra zibethica Muskrat Eutamias minimus Least chipmunk Erethizontidae (New World porcupines) Erethizon dorsatum Porcupine Muridae (Old World mice) Rattus norvegicus Norway rat 1 Mammals – Columbia NWR Family Genus Species Common Name Mus musculus House mouse Canidae (Dogs & wolves) Canis latrans Coyote Procyonidae (Raccoons) Procyon lotor Raccoon frenata Long-tailed weasel Mustela vison Mink Mustelidae (Weasels) Lutra canadensis River otter Taxidea taxus Badger Mephitis mephitis Striped skunk Lynx rufus Bobcat Felidae (Cats) Felis concolor Mountain lion hemionus Mule deer Odocoileus Cervidae (Deer) virginianus White-tailed deer Cervus elaphus Rocky Mountain elk 2. -

Quaternary Murid Rodents of Timor Part I: New Material of Coryphomys Buehleri Schaub, 1937, and Description of a Second Species of the Genus
QUATERNARY MURID RODENTS OF TIMOR PART I: NEW MATERIAL OF CORYPHOMYS BUEHLERI SCHAUB, 1937, AND DESCRIPTION OF A SECOND SPECIES OF THE GENUS K. P. APLIN Australian National Wildlife Collection, CSIRO Division of Sustainable Ecosystems, Canberra and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) K. M. HELGEN Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution, Washington and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 341, 80 pp., 21 figures, 4 tables Issued July 21, 2010 Copyright E American Museum of Natural History 2010 ISSN 0003-0090 CONTENTS Abstract.......................................................... 3 Introduction . ...................................................... 3 The environmental context ........................................... 5 Materialsandmethods.............................................. 7 Systematics....................................................... 11 Coryphomys Schaub, 1937 ........................................... 11 Coryphomys buehleri Schaub, 1937 . ................................... 12 Extended description of Coryphomys buehleri............................ 12 Coryphomys musseri, sp.nov.......................................... 25 Description.................................................... 26 Coryphomys, sp.indet.............................................. 34 Discussion . .................................................... -
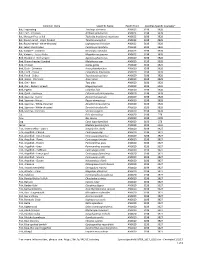
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Chapter 5 a New Species of Reithrodontomys, Subgenus
Chapter 5 A New Species of Reithrodontomys, Subgenus Aporodon (Cricetidae: Neotominae), from the Highlands of Costa Rica, with Comments on Costa Rican and Panamanian Reithrodontomys ALFRED L. GARDNER1 AND MICHAEL D. CARLETON2 ABSTRACT A new species of the rodent genus Reithrodontomys (Cricetidae: Neotominae) is described from Cerro Asuncio´n in the western Cordillera de Talamanca, Costa Rica. The long tail, elongate rostrum, bulbous braincase, and complex molars of the new species associate it with members of the subgenus Aporodon, tenuirostris species group. In its diminutive size and aspects of cranial shape, the new species (Reithrodontomys musseri, sp. nov.) most closely resembles R. microdon, a form known from highlands in Guatemala and Chiapas, Mexico. In the courseof differentially diagnosing the new species, we necessarily reviewed the Costa Rican and Panamanian subspecies of R. mexicanus based on morphological comparisons, study of paratypes and vouchers used in recent molecular studies, and morphometric analyses. We recognize Reithrodontomys cherrii (Allen, 1891) and R. garichensis Enders and Pearson, 1940, as valid species, and allocate R. mexicanus potrerograndei Goodwin, 1945, as a subjective synonym of R. brevirostris Goodwin, 1943. Critical review of museum specimens collected subsequent to Hooper’s (1952) revision is needed and would do much to improve understanding of Reithrodontomys taxonomy and distribution in Middle America. INTRODUCTION unknown to the senior author at the time. Among the mammals collected in Costa Several days of trapping at the collecting site Rica during 1966 and 1967, when Gardner and elsewhere on the Cerro Buenavista massif held an appointment with the Louisiana State over the next five months failed to produce University International Center for Medical additional specimens. -

Micheal L. Dent Richard R. Fay Arthur N. Popper Editors Rodent Bioacoustics Springer Handbook of Auditory Research
Springer Handbook of Auditory Research Micheal L. Dent Richard R. Fay Arthur N. Popper Editors Rodent Bioacoustics Springer Handbook of Auditory Research Volume 67 Series Editor Richard R. Fay, Ph.D., Loyola University Chicago, Chicago, IL, USA Arthur N. Popper, Ph.D., University of Maryland, College Park, MD, USA Editorial Board Karen Avraham, Ph.D., Tel Aviv University, Israel Andrew Bass, Ph.D., Cornell University Lisa Cunningham, Ph.D., National Institutes of Health Bernd Fritzsch, Ph.D., University of Iowa Andrew Groves, Ph.D., Baylor University Ronna Hertzano, M.D., Ph.D., School of Medicine, University of Maryland Colleen Le Prell, Ph.D., University of Texas, Dallas Ruth Litovsky, Ph.D., University of Wisconsin Paul Manis, Ph.D., University of North Carolina Geoffrey Manley, Ph.D., University of Oldenburg, Germany Brian Moore, Ph.D., Cambridge University, UK Andrea Simmons, Ph.D., Brown University William Yost, Ph.D., Arizona State University More information about this series at http://www.springer.com/series/2506 The ASA Press The ASA Press imprint represents a collaboration between the Acoustical Society of America and Springer dedicated to encouraging the publication of important new books in acoustics. Published titles are intended to reflect the full range of research in acoustics. ASA Press books can include all types of books published by Springer and may appear in any appropriate Springer book series. Editorial Board Mark F. Hamilton (Chair), University of Texas at Austin James Cottingham, Coe College Diana Deutsch, University of California, San Diego Timothy F. Duda, Woods Hole Oceanographic Institution Robin Glosemeyer Petrone, Threshold Acoustics William M. -

Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 15 1 Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys by Emmet T. Hooper & Michael D. Carleton Ann Arbor MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN August 27, 1976 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN FRANCIS C. EVANS. EDITOR The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 19 13, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 15 1 Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys by Emmet T. -

Life History and Habits of Grasshopper Mice, Genus Onychomys
112 8 2 5 : ~lli 2.8 11"12.~ I~ 11 1 . 11111 . 1.0 1.0 I.ii "= Ii,g I~ I Ii,g I~ 2.2 ~ 36 I.:.l L:: 1 L:: W iii - Wi E ~ E ~ 1.1 I.:."' ...... 1.1 I.:."' ...... 8 -- 111111 - 111111.8 111111.25 11111 1.4 11111 1.6 111111.25 11111 1.4 111111.6 MICROCOPY RESOLUTION TEST CHART MICROCOPY RESOLUTION TEST CHART N?.TIONAL BUReAU or SlANDARDS·i96.l A N~T;ONAL BUR[AU Of ST.AI';DARDS·l96J·A l;;,~o ":l ~..).;'" I H TECHNICAL BULLETIN N,.,.145 November, 1929 UNITED STATES DEPARTMENT OF AGRICULTURE WASHINGTON, D.·C. LIFE HISTORY AND HABITS OF GRASS HOPPER MICE, GENUS ONYCHOMYS By VERNON BAILEY, Senior Biologist, DWi8ion of BioZogica~ Inve8tigationa; and CHARLES C. SI;ERRY, A8sistant Biologist, DivisiOl~ of FoolJJ Hab#s Research; BUl'ep.u of Bi{)logical Survey 1 CONTENTS Pago Page Part 1. General account , _______________ .____ 1 Introductlon_ __ _________________________ 1 Part 2.chomys' Laboratory _____________________________ studies oHhe food ofOuy- _ 15 Analysis of stomach contents ___________ _ History of the genus Onychomy;____ 3 Orthoptera_________________________ _ 15 Species and subspecies of Onrchomys.. 3 Coleoptera_________________________ _ 15 General characters_______________________ <1 15 Onvchom1/8lrucoga8ter group_________ 4 16 Onvchomvs torridus group______ .____ 5 ~f~~~~il:i~::::::::::::::::::::::::: 16 Reptilia____________________________ _ 16 Miscellaueous animal food _________ _ ~~i~~~=:::::::::::::::::::::::::::::: g Vegetable food _____________________ _ 16 Variation____________________________ 6 17 General babits___________________________ 6 List of items of animal origin identi- Studies of Onychomys in capti"ity__ 6 fied in laboratory examination of the foou of Onychomys____________ 17 i5r~~':f;I~~ii=:::::::=:::::::::::::=::: ~ List of items of vegetable foou identi- Senses__ ~--------___________________ 8 fied in laboratory examination of Sanitatlon___________________________ 9 the food of Onychomys____________ 18 [~~:~~chs~~~~s:..=:::::::::::::::::::::: Ig Conclusionstions_ __ ________________________ from laboratory investiga _______ 19 l. -

Approximately 220 Species of Wild Mammals Occur in California And
Mammals pproximately 220 species of wild mammals occur in California and the surrounding waters (including introduced species, but not domestic species Asuch as house cats). Amazingly, the state of California has about half of the total number of species that occur on the North American continent (about 440). In part, this diversity reflects the sheer number of different habitats available throughout the state, including alpine, desert, coniferous forest, grassland, oak woodland, and chaparral habitat types, among others (Bakker 1984, Schoenherr 1992, Alden et al. 1998). About 17 mammal species are endemic to California; most of these are kangaroo rats, chipmunks, and squirrels. Nearly 25% of California’s mammal species are either known or suspected to occur at Quail Ridge (Appendix 9). Species found at Quail Ridge are typical of both the Northwestern California and Great Central Valley mammalian faunas. Two California endemics, the Sonoma chipmunk (see Species Accounts for scientific names) and the San Joaquin pocket mouse, are known to occur at Quail Ridge. None of the mammals at Quail Ridge are listed as threatened or endangered by either the state or federal governments, although Townsend’s big-eared bat, which is suspected to occur at Quail Ridge, is a state-listed species of special concern. Many mammal species are nocturnal, fossorial, fly, or are otherwise difficult to observe. However, it is still possible to detect the presence of mammals at Quail Ridge, both visually and by observation of their tracks, scat, and other sign. The mammals most often seen during the day are mule deer and western gray squirrels. -

SOCIAL ORGANIZATION of a SPECIES of SINGING MOUSE, Scotinomys Xerampelinus
SOCIAL ORGANIZATION OF A SPECIES OF SINGING MOUSE, Scotinomys xerampelinus By DIMITRI VINCENT BLONDEL A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2006 Copyright 2006 by Dimitri Vincent Blondel This thesis is dedicated to my parents, Pierre and Linda, who have supported me in all of my dreams and ambitions throughout my life. ACKNOWLEDGMENTS I would like to thank my committee members, Dr. Steven M. Phelps, Dr. H. Jane Brockmann, and Dr. Lyn Branch, for all of their help with this project. Dr. Brockmann and Dr. Phelps were co-chairs of my committee, and provided extremely valuable advice and support. Dr. Phelps introduced me to singing mice, the wonderful country of Panama, and ceviche, and provided generous funding and equipment support. Jorge Pino, our research assistant in the field, contributed greatly to this project. Dr. Rafael Samudio provided much appreciated logistical support with permits and other field arrangements in Panama. Thanks go to Dr. Jerry Wolff for providing radio-tracking training in Memphis, and for advice and discussion. Thanks go to Dr. Alex Ophir for help with statistics and graphs. Thanks go to Dr. Donald Dewsbury for sharing his knowledge of Scotinomys. The graduate students and post-doctoral associates in the Department of Zoology provided valued input; this includes Polly Campbell, Ondi Crino, Billy Gunnels, Joanna Matos, Toshi Okuyama, and many others. Thanks go to Dr. Mel Sunquist and Dr. Stephen Coates for providing my initial radio-tracking and live-trapping training in their field techniques course at the Ordway Preserve. -

Mammals of Colorado, Second Edition
1 Environments of Colorado Mammals are a familiar and important component of understand the distribution and abundance of mammals Earth’s biodiversity. Biodiversity is the kinds of organisms and the details of their daily lives we must fi rst understand and their genetic and ecological relationships—an evolu- the resource base, the mosaic of Colorado’s environments tionary and ecological phenomenon in space and time (E. in space and time. Wilson 1992). The mammalian fauna of Colorado is a fas- cinating piece of that whole. To understand the diversity of mammals we need to have a perspective of the ecosphere more generally. Such a perspective is the purpose of this Geography chapter, with a focus on environments of Colorado. Colorado is known for its scenic beauty—from majes- From the standpoint of political geography, Colorado is tic mountain peaks and rushing white rivers tumbling simple: it is roughly rectangular (if we neglect some minor down dark canyons, to red-rock deserts and ceaselessly old surveyors’ errors and the fact that Earth is spherical), shifting sand dunes, to the expansive sweep of the short- measuring approximately 607 km by 444 km (377 by 276 grass prairie. Grandeur is wherever we stop to appreciate mi.) and encompassing some 270,000 km2 (104,000 sq. mi.). it, at every scale, from canyons carved in crystalline rocks Colorado lies between approximately 102° and 109° west 2 billion years old, to bold peaks sculpted by the glaciers longitude and 37° and 41° north latitude, and is subdi- of the last Ice Age, to last night’s furtive trail of a mouse vided into 64 counties (Map 1-1). -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34.