Aeromonas Salmonicida Activates Rainbow Trout Igm+ B Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TLR3-Dependent Activation of TLR2 Endogenous Ligands Via the Myd88 Signaling Pathway Augments the Innate Immune Response
cells Article TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response 1 2, 1 3 Hellen S. Teixeira , Jiawei Zhao y, Ethan Kazmierski , Denis F. Kinane and Manjunatha R. Benakanakere 2,* 1 Department of Orthodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA 19004, USA; [email protected] (H.S.T.); [email protected] (E.K.) 2 Department of Periodontics, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA 19004, USA; [email protected] 3 Periodontology Department, Bern Dental School, University of Bern, 3012 Bern, Switzerland; [email protected] * Correspondence: [email protected] Present address: Department of Pathology, Wayne State University School of Medicine, y 541 East Canfield Ave., Scott Hall 9215, Detroit, MI 48201, USA. Received: 30 June 2020; Accepted: 12 August 2020; Published: 17 August 2020 Abstract: The role of the adaptor molecule MyD88 is thought to be independent of Toll-like receptor 3 (TLR3) signaling. In this report, we demonstrate a previously unknown role of MyD88 in TLR3 signaling in inducing endogenous ligands of TLR2 to elicit innate immune responses. Of the various TLR ligands examined, the TLR3-specific ligand polyinosinic:polycytidylic acid (poly I:C), significantly induced TNF production and the upregulation of other TLR transcripts, in particular, TLR2. Accordingly, TLR3 stimulation also led to a significant upregulation of endogenous TLR2 ligands mainly, HMGB1 and Hsp60. By contrast, the silencing of TLR3 significantly downregulated MyD88 and TLR2 gene expression and pro-inflammatory IL1β, TNF, and IL8 secretion. The silencing of MyD88 similarly led to the downregulation of TLR2, IL1β, TNF and IL8, thus suggesting MyD88 / to somehow act downstream of TLR3. -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

NF-Κb Signaling and Its Relevance to the Treatment of Mantle Cell Lymphoma
Balaji et al. Journal of Hematology & Oncology (2018) 11:83 https://doi.org/10.1186/s13045-018-0621-5 REVIEW Open Access NF-κB signaling and its relevance to the treatment of mantle cell lymphoma Swathi Balaji, Makhdum Ahmed, Elizabeth Lorence, Fangfang Yan, Krystle Nomie and Michael Wang* Abstract Mantle cell lymphoma is an aggressive subtype of non-Hodgkin B cell lymphoma that is characterized by a poor prognosis determined by Ki67 and Mantle Cell International Prognostic Index scores, but it is becoming increasingly treatable. The majority of patients, especially if young, achieve a progression-free survival of at least 5 years. Mantle cell lymphoma can initially be treated with an anti-CD20 antibody in combination with a chemotherapy backbone, such as VR-CAP (the anti-CD20 monoclonal antibody rituximab administered with cyclophosphamide, doxorubicin, and prednisone) or R-CHOP (the anti-CD20 monoclonal antibody rituximab administered with cyclophosphamide, doxorubicin, vincristine, and prednisone). While initial treatment can facilitate recovery and complete remission in a few patients, many patients experience relapsed or refractory mantle cell lymphoma within 2 to 3 years after initial treatment. Targeted agents such as ibrutinib, an inhibitor of Bruton’s tyrosine kinase, which has been approved only in the relapsed setting, can be used to treat patients with relapsed or refractory mantle cell lymphoma. However, mantle cell lymphoma cells often acquire resistance to such targeted agents and continue to survive by activating alternate signaling pathways such as the PI3K-Akt pathway or the NF-κB pathways. NF-κB is a transcription factor family that regulates the growth and survival of B cells; mantle cell lymphoma cells depend on NF-κB signaling for continued growth and proliferation. -
Recognition of Lipopolysaccharide Pattern by TLR4 Complexes
OPEN Experimental & Molecular Medicine (2013) 45, e66; doi:10.1038/emm.2013.97 & 2013 KSBMB. All rights reserved 2092-6413/13 www.nature.com/emm REVIEW Recognition of lipopolysaccharide pattern by TLR4 complexes Beom Seok Park1 and Jie-Oh Lee2 Lipopolysaccharide (LPS) is a major component of the outer membrane of Gram-negative bacteria. Minute amounts of LPS released from infecting pathogens can initiate potent innate immune responses that prime the immune system against further infection. However, when the LPS response is not properly controlled it can lead to fatal septic shock syndrome. The common structural pattern of LPS in diverse bacterial species is recognized by a cascade of LPS receptors and accessory proteins, LPS binding protein (LBP), CD14 and the Toll-like receptor4 (TLR4)–MD-2 complex. The structures of these proteins account for how our immune system differentiates LPS molecules from structurally similar host molecules. They also provide insights useful for discovery of anti-sepsis drugs. In this review, we summarize these structures and describe the structural basis of LPS recognition by LPS receptors and accessory proteins. Experimental & Molecular Medicine (2013) 45, e66; doi:10.1038/emm.2013.97; published online 6 December 2013 Keywords: lipopolysaccharide (LPS); Toll-like receptor 4 (TLR4); MD-2; CD14; LBP INTRODUCTION the cell surface by a glycosylphosphatidylinositol anchor. CD14 In the initial phase of infection, the innate immune system splits LPS aggregates into monomeric molecules and presents generates a rapid inflammatory response that blocks the them to the TLR4–MD-2 complex. Aggregation of the TLR4– growth and dissemination of the infectious agent. -

Integrin Αvβ6-EGFR Crosstalk Regulates Bidirectional Force Transmission and Controls Breast Cancer Invasion
bioRxiv preprint doi: https://doi.org/10.1101/407908; this version posted September 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Integrin αVβ6-EGFR crosstalk regulates bidirectional force transmission and controls breast cancer invasion Joanna R. Thomas1#, Kate M. Moore2#, Caroline Sproat2, Horacio J. Maldonado-Lorca1, Stephanie Mo1, Syed Haider3, Dean Hammond1, Gareth J. Thomas5, Ian A. Prior1, Pedro R. Cutillas2, Louise J. Jones2, John F. Marshall2†, Mark R. Morgan1† 1 Institute of Translational Medicine, University of Liverpool, Crown Street, Liverpool, L69 3BX, UK. 2 Centre for Tumour Biology, Barts Cancer Institute, Queen Mary University London, John Vane Science Centre, Charterhouse Square, London EC1M 6BQ, UK. 3 The Weatherall Institute of Molecular Medicine, Department of Oncology, University of Oxford, Oxford OX3 9DS, UK. 4 Cancer Research UK Centre for Epidemiology, Mathematics and Statistics, Wolfson Institute of Preventative Medicine, Queen Mary University London, Charterhouse Square, London EC1M 6BQ, UK. 5 Cancer Sciences Division, Somers Building, Southampton General Hospital, Southampton, SO16 6YA, UK. # Denotes equal contribution † Corresponding author Correspondence to: Dr Mark R. Morgan, PhD, Cellular & Molecular Physiology, Institute of Translational Medicine, University of Liverpool, Crown Street, Liverpool, L69 3BX, -

A CROSS-SECTIONAL STUDY on the EFFECT of HIV VIRION and BACTERIAL LPS on MEMORY B CELL APOPTOSIS by WEI JIANG Submitted in Part
A CROSS-SECTIONAL STUDY ON THE EFFECT OF HIV VIRION AND BACTERIAL LPS ON MEMORY B CELL APOPTOSIS by WEI JIANG Submitted in partial fulfillment of requirements for the degree of Master of Science Dissertation Advisor: Dr. Daniel Tisch Department of Epidemiology and Biostatistics CASE WESTEN RESERVE UNIVERSITY May, 2012 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Wei Jiang candidate for the Master of Science degree*. (signed) Daniel Tisch (chair of the committee) Daniel Tisch XiaoFeng Zhu Mark D. Schluchter (date) March27th, 2012 *We also certify that written approval has been obtained for any proprietary material contained therein. 2 Dedicated to my dear husband Zhuang Wan whose patience and encouragement have always given me great support 3 I very much appreciate Dr. PingFu Fu for his support and guidance. I would like to acknowledge my advisor Dr. Daniel Tisch, and committee members Dr. Mark D. Schluchter and Dr. XiaoFeng Zhu. This work was supported by STERIS grant at Case Western Reserve University and NIAID R01AI091526. 4 TABLE OF CONTENTS Abstract 9 Introduction 11 Specific aims 20 Study methods 21 Results 36 Discussion 59 References 66 5 LIST OF TABLES Table 1. Characteristics of HIV-negative control and 36 HIV-infected subjects Table 2. Dependent variable and independent variables 37 Table 3. Correlation test in all subjects 46 Table 4. Correlation test in control subjects 46 Table 5. Correlation test in HIV-infected subjects 47 Table 6. Independent variables include plasma level of FasL, 48 Fas geometric mean expression on memory B cells, or HIV status among all subjects in a linear regression model by forward selection Table 7. -
Reviewed by HLDA1
Human CD Marker Chart Reviewed by HLDA1 T Cell Key Markers CD3 CD4 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD8 CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD74 DHLAG, HLADG, Ia-g, li, invariant chain HLA-DR, CD44 + + + + + + CD158g KIR2DS5 + + CD248 TEM1, Endosialin, CD164L1, MGC119478, MGC119479 Collagen I/IV Fibronectin + ST6GAL1, MGC48859, SIAT1, ST6GALL, ST6N, ST6 b-Galactosamide a-2,6-sialyl- CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD75 CD22 CD158h KIR2DS1, p50.1 HLA-C + + CD249 APA, gp160, EAP, ENPEP + + tranferase, Sialo-masked lactosamine, Carbohydrate of a2,6 sialyltransferase + – – + – – + – – CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD75S a2,6 Sialylated lactosamine CD22 (proposed) + + – – + + – + + + CD158i KIR2DS4, p50.3 HLA-C + – + CD252 TNFSF4, -

Role of TLR1, TLR2 and TLR6 in the Modulation of Intestinal
Choteau et al. Gut Pathog (2017) 9:9 DOI 10.1186/s13099-017-0158-0 Gut Pathogens RESEARCH Open Access Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination Laura Choteau1,2,3, Hélène Vancraeyneste1,2,3, Didier Le Roy4, Laurent Dubuquoy1,2, Luiginia Romani5, Thierry Jouault1,2, Daniel Poulain1,2,3, Boualem Sendid1,2,3, Thierry Calandra4, Thierry Roger4 and Samir Jawhara1,2,3* Abstract Background: Toll-like receptors (TLRs) are the major pattern recognition receptors that mediate sensing of a wide range of microorganisms. TLR2 forms heterodimers with either TLR1 or TLR6, broadening its ligand diversity against pathogens. TLR1, TLR2 and TLR6 have been implicated in the recognition of Candida albicans, an opportunistic fungal pathogen that colonizes the gastrointestinal tract. In this study, we explored whether the deficiency in TLR1, TLR2 or TLR6 impacts C. albicans colonization and inflammation-associated colonic injury in the dextran sulfate sodium (DSS)- induced colitis in mice. Results: DSS treatment and C. albicans challenge induced greater weight loss, worse clinical signs of inflammation, / / / higher histopathologic scores, and increased mortality rates in TLR1− − and TLR2− − mice when compared to TLR6− − / and wild-type mice. The number of C. albicans colonies in the stomach, colon and feces was decreased in TLR6− − / / mice as compared to TLR2− −, TLR1− − and wild-type mice. Interestingly, the population of E. coli in colonic luminal / contents, intestinal permeability to FITC-dextran and cytokine expression were significantly increased in TLR1− − and / / TLR2− − mice, while they were decreased in TLR6− − mice. Conclusion: In contrast to TLR6, both TLR1 and TLR2 deficiencies increased intestinal inflammation, and the over- growth of C. -

Transcriptomic Response of Breast Cancer Cells to Anacardic Acid David J
www.nature.com/scientificreports OPEN Transcriptomic response of breast cancer cells to anacardic acid David J. Schultz1, Abirami Krishna2, Stephany L. Vittitow2, Negin Alizadeh-Rad2, Penn Muluhngwi2, Eric C. Rouchka 3 & Carolyn M. Klinge 2 Received: 5 December 2017 Anacardic acid (AnAc), a potential dietary agent for preventing and treating breast cancer, inhibited Accepted: 10 May 2018 the proliferation of estrogen receptor α (ERα) positive MCF-7 and MDA-MB-231 triple negative Published: xx xx xxxx breast cancer cells. To characterize potential regulators of AnAc action, MCF-7 and MDA-MB-231 cells were treated for 6 h with purifed AnAc 24:1n5 congener followed by next generation transcriptomic sequencing (RNA-seq) and network analysis. We reported that AnAc-diferentially regulated miRNA transcriptomes in each cell line and now identify AnAc-regulated changes in mRNA and lncRNA transcript expression. In MCF-7 cells, 80 AnAc-responsive genes were identifed, including lncRNA MIR22HG. More AnAc-responsive genes (886) were identifed in MDA-MB-231 cells. Only six genes were commonly altered by AnAc in both cell lines: SCD, INSIG1, and TGM2 were decreased and PDK4, GPR176, and ZBT20 were increased. Modeling of AnAc-induced gene changes suggests that AnAc inhibits monounsaturated fatty acid biosynthesis in both cell lines and increases endoplasmic reticulum stress in MDA-MB-231 cells. Since modeling of downregulated genes implicated NFκB in MCF-7, we confrmed that AnAc inhibited TNFα-induced NFκB reporter activity in MCF-7 cells. These data identify new targets and pathways that may account for AnAc’s anti-proliferative and pro-apoptotic activity. -

Aspergillosis Tlrs Govern Neutrophil Activity In
TLRs Govern Neutrophil Activity in Aspergillosis Silvia Bellocchio, Silvia Moretti, Katia Perruccio, Francesca Fallarino, Silvia Bozza, Claudia Montagnoli, Paolo Mosci, This information is current as Grayson B. Lipford, Lucia Pitzurra and Luigina Romani of September 28, 2021. J Immunol 2004; 173:7406-7415; ; doi: 10.4049/jimmunol.173.12.7406 http://www.jimmunol.org/content/173/12/7406 Downloaded from References This article cites 60 articles, 26 of which you can access for free at: http://www.jimmunol.org/content/173/12/7406.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2004 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology TLRs Govern Neutrophil Activity in Aspergillosis1 Silvia Bellocchio,* Silvia Moretti,* Katia Perruccio,* Francesca Fallarino,* Silvia Bozza,* Claudia Montagnoli,* Paolo Mosci,* Grayson B. Lipford,† Lucia Pitzurra,* and Luigina Romani2* Polymorphonuclear neutrophils (PMNs) are essential in initiation and execution of the acute inflammatory response and subse- quent resolution of fungal infection. -

Viruses and Gram-Positive Bacteria TLR Profile and Differentially
Human Langerhans Cells Express a Specific TLR Profile and Differentially Respond to Viruses and Gram-Positive Bacteria This information is current as Vincent Flacher, Marielle Bouschbacher, Estelle Verronèse, of September 30, 2021. Catherine Massacrier, Vanja Sisirak, Odile Berthier-Vergnes, Blandine de Saint-Vis, Christophe Caux, Colette Dezutter-Dambuyant, Serge Lebecque and Jenny Valladeau J Immunol 2006; 177:7959-7967; ; Downloaded from doi: 10.4049/jimmunol.177.11.7959 http://www.jimmunol.org/content/177/11/7959 References This article cites 54 articles, 18 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/177/11/7959.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 30, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Human Langerhans Cells Express a Specific TLR Profile and Differentially Respond to Viruses and Gram-Positive Bacteria1 Vincent Flacher,2* Marielle Bouschbacher,† Estelle Verrone`se,† Catherine Massacrier,* Vanja Sisirak,‡ Odile Berthier-Vergnes,† Blandine de Saint-Vis,* Christophe Caux,*‡ Colette Dezutter-Dambuyant,† Serge Lebecque,3* and Jenny Valladeau4† Dendritic cells (DC) are APCs essential for the development of primary immune responses. -
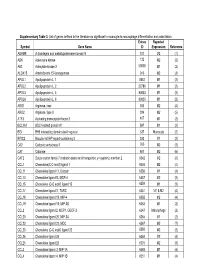
Supplementary Table 3. List of Genes Defined in the Literature As Significant in Monocyte-To-Macrophage Differentiation and Polarization
Supplementary Table 3. List of genes defined in the literature as significant in monocyte-to-macrophage differentiation and polarization. Entrez Reported Symbol Gene Name ID Expression Reference ADAM8 A disintegrin and metalloproteinase domain 8 101 M2 (1) ADK Adenosine kinase 132 M2 (2) AK3 Adenylate kinase 3 50808 M1 (2) ALOX15 Arachidonate 15-lipoxygenase 246 M2 (3) APOL1 Apolipoprotein L, 1 8542 M1 (2) APOL2 Apolipoprotein L, 2 23780 M1 (2) APOL3 Apolipoprotein L, 3 80833 M1 (2) APOL6 Apolipoprotein L, 6 80830 M1 (2) ARG1 Arginase, liver 383 M2 (4) ARG2 Arginase, type II 384 M2 (5) ATF3 Activating transcription factor 3 467 M1 (2) BCL2A1 BCL2-related protein A1 597 M1 (2) BID BH3 interacting domain death agonist 637 Monocyte (2) BIRC3 Baculoviral IAP repeat-containing 3 330 M1 (2) CA2 Carbonic anhydrase II 760 M2 (2) CAT Catalase 847 M2 (6) CAT2 Solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 6542 M2 (6) CCL1 Chemokine(C-C motif) ligand 1 6346 M2 (4) CCL11 Chemokine ligand 11, Eotaxin 6356 M1 (4) CCL13 Chemokine ligand13, MCP-4 6357 M2 (2) CCL15 Chemokine (C-C motif) ligand 15 6359 M1 (2) CCL17 Chemokine ligand17, TARC 6361 M1 & M2 (4) CCL18 Chemokine ligand 18, MIP-4 6362 M2 (4) CCL19 Chemokine ligand 19, MIP-3B 6363 M1 (2) CCL2 Chemokine ligand 2, MCP1, GDCF-2 6347 Macrophage (2) CCL20 Chemokine ligand 20, MIP-3A 6364 M1 (2) CCL22 Chemokine ligand 22, MDC 6367 M2 (7) CCL23 Chemokine (C-C motif) ligand 23 6368 M2 (2) CCL24 Chemokine ligand 24 6369 M2 (4) CCL25 Chemokine ligand 25 6370 M2 (8) CCL3 Chemokine