Opportunities and Challenges in Exploring Indian Non-Mulberry Silk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State : Assam S. No. Name of Awardee/ Weaver /Co-Op Society
State : Assam S. Name of Complete District Photo of Weaver Award Name of Indicat Weave Technique of Photos of products Produ No. Awardee/ address M.No. received, exclusive e if it is /s product ct e-mail if any handloom GI practic weaving Descr Weaver product produc ed in iption t the /Co-op handlo Society om etc. produc t 1 Sh. Hiralal Vill - Kalita Kamru - Muga Silk Yes Plain Kalita Para, PO / PS - p (R ) Chadar- (Muga weave Sualkuchi, Mekhela Silk) Assam, Pin - 781103 Mob No.- 9954181623 Anne xed separ 2 M/s Assam No.1, Bapuji Kamru Muga Silk - Plain ately Samabay Path, p (R), Chadar- weave (Ann Resham Sualkuchi, Mekhela exure Assam, Pin- Treadle, Pratisthan I) 781103 Jacquard, Ltd. Mob.- extra weft 9435417633 3 Smt. Bina Vill. & PO- Kamru National No Kalita Bijoynagar, p (R ) Award - Distt.- Kamrup 2008 Eri Silk Shawl, (R),Assam- 781122, Mob.- 8486356009 4 Smt. Hiran W/o Anil Kamru No Goswami Goswami, Vill – p (R ) Dahali (Gohai Chadar – Medhi Para), Mekhela PO – Batarhat (Rampur), Pin – National 7831122 Award - Mob – 2012 9435365953 5 Smt. Anju Jakir Hussain Kamru Mulberry Silk No Buragohai Road, p (M) National Chadar n Sarumotaria, Merit Kalaguru Path, Certificat By Lane No. 8, e Guwahati, (Kamala Assam Devi Treadle, Pin-781036 Chattop Jacquard, Mob. – Plain Anne adhyay) - extra weft 8638032117 / weave xed 9435114805 2018 Sepa rately 6 Smt. Resham Kamru National Eri Silk Stole No (Ann Anuradha Nagar, p (M) Award - (Naturally exure Kuli Pegu Khanapara, 2009 dyed) I) Guwahati, Kamrup (M), Assam, Mob no. – 8761959272 / 9864075276 7 M/s Ava Matia Hills, Kamru Eri Silk Stole No Creation Kahikuchi, p (M) Guwahati,Ka mrup (M),Assam, mob no. -
Lower New Colony, Shillong-793003, Meghalaya (India) STD: 0364, Web: Neepco.Co.In
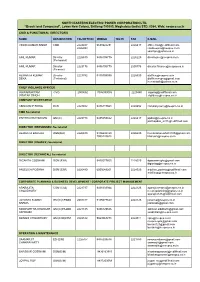
NORTH EASTERN ELECTRIC POWER CORPORATION LTD. “Brook land Compound”, Lower New Colony, Shillong-793003, Meghalaya (India) STD: 0364, Web: neepco.co.in CMD & FUNCTIONAL DIRECTORS NAME DESIGNATION TEL(OFFICE) MOBILE TEL(R) FAX E-MAIL VINOD KUMAR SINGH CMD 2224487 9650922231 2226417 [email protected] 2226453 [email protected] [email protected] ANIL KUMAR Director 2226630 9436105775 2226225 [email protected] (Personnel) ANIL KUMAR Director 2223176 9436105775 2505776 [email protected] (Finance) HEMANTA KUMAR Director 2227792 9436709095 2228520 [email protected] DEKA (Technical) [email protected] [email protected] CHIEF VIGILANCE OFFICER KHWAIRAKPAM CVO 2503652 7086099086 2229450 [email protected] PRATAP SINGH [email protected] COMPANY SECRETARIAT ABINOAM P RONG DCS 2228652 9436117663 2228652 [email protected] CMD Secretariat PARTHA PRATIM DAS GM (C) 2229778 9435559842 2226417 [email protected] [email protected] DIRECTOR (PERSONNEL) Secretariat HEMANTA BARUAH DGM(HR) 2226630 9436632420 2226225 [email protected] 7005120618 [email protected] DIRECTOR (FINANCE) Secretariat DIRECTOR (TECHNICAL) Secretariat DIGANTA GOSWAMI DGM (E/M) 9435577655 2228520 [email protected] [email protected] ANJELICA POSHNA DGM (E/M) 2226480 6009249201 2228520 [email protected] anjelicap@ neepco.co.in CORPORATE PLANNING & BUSINESS DEVELOPMENT / CORPORATE PROJECT MANAGEMENT APARAJITA CGM (Civil) 2221737 9436303944 2222126 [email protected] CHOUDHURY -

Dimasa Kachari of Assam
ETHNOGRAPHIC STUDY NO·7II , I \ I , CENSUS OF INDIA 1961 VOLUME I MONOGRAPH SERIES PART V-B DIMASA KACHARI OF ASSAM , I' Investigation and Draft : Dr. p. D. Sharma Guidance : A. M. Kurup Editing : Dr. B. K. Roy Burman Deputy Registrar General, India OFFICE OF THE REGISTRAR GENERAL, INDIA MINISTRY OF HOME AFFAIRS NEW DELHI CONTENTS FOREWORD v PREFACE vii-viii I. Origin and History 1-3 II. Distribution and Population Trend 4 III. Physical Characteristics 5-6 IV. Family, Clan, Kinship and Other Analogous Divisions 7-8 V. Dwelling, Dress, Food, Ornaments and Other Material Objects distinctive qfthe Community 9-II VI. Environmental Sanitation, Hygienic Habits, Disease and Treatment 1~ VII. Language and Literacy 13 VIII. Economic Life 14-16 IX. Life Cycle 17-20 X. Religion . • 21-22 XI. Leisure, Recreation and Child Play 23 XII. Relation among different segments of the community 24 XIII. Inter-Community Relationship . 2S XIV Structure of Soci141 Control. Prestige and Leadership " 26 XV. Social Reform and Welfare 27 Bibliography 28 Appendix 29-30 Annexure 31-34 FOREWORD : fhe Constitution lays down that "the State shall promote with special care the- educational and economic hterest of the weaker sections of the people and in particular of the Scheduled Castes and Scheduled Tribes and shall protect them from social injustice and all forms of exploitation". To assist States in fulfilling their responsibility in this regard, the 1961 Census provided a series of special tabulations of the social and economic data on Scheduled Castes and Scheduled Tribes. The lists of Scheduled Castes and Scheduled Tribes are notified by the President under the Constitution and the Parliament is empowered to include in or exclude from the lists, any caste or tribe. -

Students Details 2Nd Year
Students details 2nd year S.L NAME OF THE STUDENT ADMITTED FATHERS NAME ADDRESS CATEGORY YEAR OF RESULT PERCE CONTACT ADMISSION (GEN/SC/S ADMISSION NTAGE NO/MOBILE FEE.(RECEIPT NO. T/OBC) NO DATE& AMOUNT) 1 SMRITI BHATTACHARYA SUJIT KUMAR VILL:S.M ROAD,P.P GEN 2016 First 73.57 789662817 4653,50000 BHATTACHARYA PATH P.O:ITACHALI P.S:DO.NAGAON 2 SRIPARNA KAR LT.SUNIT KUMAR VILL:R.K ROAD,ITACHALI OBC 2016 First 65.85 9706561743 4654,50000 KAR P.O:NAGAON P.S:ITACHALI,NAGAON 3 FARHANA PARBEEN BARBHUYAN HABIB ULLAH VILL:BIBTIA NO.1 GEN 2016 First 74.28 9435161529 4663,50000 BARBHUYAN P.O:DHING P.S:DHING NAGAON ASAM 4 NITU KHAITAN ASHOK KHAITAN VILL:ROHA GEN 2016 First 70 8876542988 4682,50000 P.O: ROHA P.S:ROHA,NAGAON 5 NILAKHI BORA BAKUL BORA VILL:BHEAUGURI GEN 2016 First 67.42 9707028846 4629,50000 P.O:DO P.S:SAMAGURI DIST:NAGAON 6 SUSHIL MAHANTA PURNA KANTA VILL:HEMARBORI,BORA GEN 2016 First 71.28 7896472282 4675,50000 MAHANTA NGALALI P.O:DO P.S:KHATAWAL NAGAON ASSAM 7 RUP JYOTI KALITA DHARANI KALITA VILL:ATGAON GEN 2016 First 68.55 9706599051 4683,50000 P.O:DHING P.S: DHING DIST : NAGAON 8 NABA JYOTI KALITA NIRMAL KALITA VILL:TELIA GAON GEN 2016 First 70.14 8486501842 4684,50000 P.O:ITACHALI P.S:HAIBORGAON DIST: NAGAON 9 MRIGASHREE BORA TANKESWAR VILL:POLICE RESERVE OBC 2016 First 70.28 9435822668 4655,50000 BORA P.O:NAGAON P.S:SADAR PIN:782001 DIST:NAGAON 10 ADIL SHEIKH RAHMAN ALI VILL:A.T ROAD,NATUN GEN 2016 First 70.71 9706158647 4667,50000 BAZAR,ITACHALI P.O:NAGAON P.S:SADAR PIN:782001 DIST: NAGAON 11 RASMONI DAS MILAN CH. -
DATABASE of PHONE NOS. of IMPORTANT GOVT. OFFICIALS of DISTRICTS Sl DISTRICT No NAME NAME of the OFFICER DESIGNATION MOBILE NO Shri S
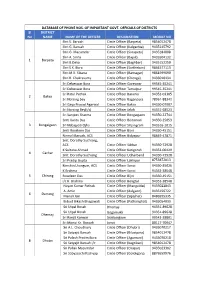
DATABASE OF PHONE NOS. OF IMPORTANT GOVT. OFFICIALS OF DISTRICTS Sl DISTRICT No NAME NAME OF THE OFFICER DESIGNATION MOBILE NO Shri S. Baruah Circle Officer (Barpeta) 9854012478 Shri G. Baruah Circle Officer (Kalgachia) 9435145792 Shri G. Mazumdar Circle Officer (Sarupeta) 9435184008 Shri A. Sinha Circle Officer (Bajali) 9435504132 1 Barpeta Shri B.Deka Circle Officer (Baghbar) 9435152250 Shri S.K. Bora Circle Officer (Sarthebari) 9864577113 Shri M.K. Sikaria Circle Officer (Barnagar) 9864599690 Shri R. Chakravarty Circle Officer (Chenga) 9435010434 Sri Debeswar Bora Circle Officer Goreswar 99545‐35241 Sri Debeswar Bora Circle Officer Tamulpur 99545‐35241 Sri Ratul Pathak Circle Officer Barama 94352‐03305 2 Baksa Sri Norsing bey Circle Officer Baganpara 78961‐88342 Sri Gaya Prasad Agarwal Circle Officer Baksa 94350‐07907 Sri Norsing Bey(i/c) Circle Officer Jalah 94351‐68523 Sri Sanjeev Sharma Circle Officer Bongaigaon 94350‐22744 Smti Kanta Das Circle Officer Boitamari 94350‐25053 3 Bongaigaon Sri Nabajyoti Ojha Circle Officer Srijangram 943516‐1015 Smti Roseleen Das Circle Officer Bijni 94350‐45151 Nirmali Baruah, ACS Circle Officer Bidyapur 98649‐47871 Smt. Dorothy Suchiang, ACS Circle Officer Silchar 94350‐72928 K.Sultana Ahmed Circle Officer Katigorah 94352‐00429 4 Cachar Smt. Dorothy Suchiang Circle Officer Udharband 94350‐72928 Sri Pradip Gupta Circle Officer Lakhipur 8753872013 Kimchin Lhangum, ACS Circle Officer Sonai 94350‐35026 K.Brahma Circle Officer Sonai 94353‐38548 5 Chirang Roseleen Das Circle Officer Bijni 94350‐45151 i/c K. Brahma Circle Officer Bengtal 94353‐38548 Nayan Kumar Pathak Circle Officer (Mangaldai) 9435022843 A. Amin Circle Officer (dalgaon) 9435156722 6 Darrang Manali Jain Circle Officer (Sipajhar) 8486595335 Bidyut Bikash Bhagawati Circle Officer (Patharighat) 9435054033 Sri Utpal Borah Dhemaji 94351‐89628 Sri Utpal Borah Gogamukh 94351‐89628 Dhemaji Sri Ranjit Konwar Sissiborgaon 99542‐28801 Sri Monui Kr. -

Empire's Garden: Assam and the Making of India
A book in the series Radical Perspectives a radical history review book series Series editors: Daniel J. Walkowitz, New York University Barbara Weinstein, New York University History, as radical historians have long observed, cannot be severed from authorial subjectivity, indeed from politics. Political concerns animate the questions we ask, the subjects on which we write. For over thirty years the Radical History Review has led in nurturing and advancing politically engaged historical research. Radical Perspec- tives seeks to further the journal’s mission: any author wishing to be in the series makes a self-conscious decision to associate her or his work with a radical perspective. To be sure, many of us are currently struggling with the issue of what it means to be a radical historian in the early twenty-first century, and this series is intended to provide some signposts for what we would judge to be radical history. It will o√er innovative ways of telling stories from multiple perspectives; comparative, transnational, and global histories that transcend con- ventional boundaries of region and nation; works that elaborate on the implications of the postcolonial move to ‘‘provincialize Eu- rope’’; studies of the public in and of the past, including those that consider the commodification of the past; histories that explore the intersection of identities such as gender, race, class and sexuality with an eye to their political implications and complications. Above all, this book series seeks to create an important intellectual space and discursive community to explore the very issue of what con- stitutes radical history. Within this context, some of the books pub- lished in the series may privilege alternative and oppositional politi- cal cultures, but all will be concerned with the way power is con- stituted, contested, used, and abused. -

History of North East India (1228 to 1947)
HISTORY OF NORTH EAST INDIA (1228 TO 1947) BA [History] First Year RAJIV GANDHI UNIVERSITY Arunachal Pradesh, INDIA - 791 112 BOARD OF STUDIES 1. Dr. A R Parhi, Head Chairman Department of English Rajiv Gandhi University 2. ************* Member 3. **************** Member 4. Dr. Ashan Riddi, Director, IDE Member Secretary Copyright © Reserved, 2016 All rights reserved. No part of this publication which is material protected by this copyright notice may be reproduced or transmitted or utilized or stored in any form or by any means now known or hereinafter invented, electronic, digital or mechanical, including photocopying, scanning, recording or by any information storage or retrieval system, without prior written permission from the Publisher. “Information contained in this book has been published by Vikas Publishing House Pvt. Ltd. and has been obtained by its Authors from sources believed to be reliable and are correct to the best of their knowledge. However, IDE—Rajiv Gandhi University, the publishers and its Authors shall be in no event be liable for any errors, omissions or damages arising out of use of this information and specifically disclaim any implied warranties or merchantability or fitness for any particular use” Vikas® is the registered trademark of Vikas® Publishing House Pvt. Ltd. VIKAS® PUBLISHING HOUSE PVT LTD E-28, Sector-8, Noida - 201301 (UP) Phone: 0120-4078900 Fax: 0120-4078999 Regd. Office: 7361, Ravindra Mansion, Ram Nagar, New Delhi – 110 055 Website: www.vikaspublishing.com Email: [email protected] About the University Rajiv Gandhi University (formerly Arunachal University) is a premier institution for higher education in the state of Arunachal Pradesh and has completed twenty-five years of its existence. -

A Study on Seasonal and Temporal Variation in Physico-Chemical and Hydrological Characteristics of River Kolong at Nagaon Town, Assam, India
Available online a twww.scholarsresearchlibrary.com Scholars Research Library Archives of Applied Science Research, 2015, 7 (5):110-117 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 A study on seasonal and temporal variation in physico-chemical and hydrological characteristics of River Kolong at Nagaon Town, Assam, India Minakshi Bora* and Dulal C. Goswami Department of Environmental Science, Gauhati University, Assam, India _____________________________________________________________________________________________ ABSTRACT Healthy natural ecosystem is an indispensable prerequisite of a hale and hearty society. Although the socio- economic, cultural as well as the political well-being of a region is directly correlated to a healthy and sustainable ecosystem, the mankind has relentlessly been tampering with these valued assets of nature for their comfort. Moreover, out of all the existing ecosystems it is a fact that aquatic ecosystem is the most imperiled one. Thus, it is the need of the hour to take some stern and state-of-the-art actions towards upholding the aquatic ecosystems of our environment. Like many other rivers of the world, the Kolong River of Assam is also going through a staid phase of degeneration as a result of human intervention, for the last half century. The objective of the present study is to reveal the ailing condition of the Kolong River along Nagaon town and to assess the change in the trend of its water quality parameters for a time interval of twenty years (i.e. 1992 to 2013). The results revealed that river discharge has diminished with time except for the peak monsoonal period. Similarly, few water quality parameters viz. -

Students Details 1St Year
Students details 1st year SL.N NAME OF THE FATHER’s NAME ADDRESS CATEGORY YEAR OF RESULT PERCENTAGE CONTACT NO/MOBILE ADMISSION O STUDENT (GEN/SC/ST/ ADMISSION NO. FEE(RECEIPT ADMITTED OBC) NO.DATE&A MOUNT) 1. PAGRAG BORAH PRABHAT BORAH VILL:KALIABOR TINIALI GEN. 2017 N/A N/A 9401703706 4505,60000 P.O :KUWARITAL P.S:KALIABOR PIN:782137 DIST:NAGAON 2. UDIPTA HAZARIKA PRABHAT HAZARIKA VILL:TARAJAR SONARIGAON GEN. 2017 N/A N/A 8876813440 4506,60000 P.O:JORHAT P.S:PULIBOR PIN:785001 DIST:JORHAT 3. MD. RASHIDUL EUSUP ALI VILL:KUMARGAON GEN. 2017 N/A N/A 9864891686 4507,60000 HOQUE P.O:KUMAR P.S:RUPOHI HAT PIN:782125 DIST:NAGAON 4. GARIMA PHUKAN GOPAL CH. PHUKAN VILL:SENCHOWA OBC. 2017 N/A N/A 9101024067 4509,60000 P.O:SENCHOWA P.S:NAGAON PIN:782002 DIST:NAGAON 5. ANIRBAN NANDY ARUN BIKASH NANDI VILL:BRICKFIELD COLONY GEN. 2017 N/A N/A 8724021964 4600,60000 P.O:LUMDING P.S:LUMDING PIN:782447 DIST:HOJAI 6. RUBI BARUAH JATIN BARUAH VILL:NAMATI DIHINGIA GAON OBC. 2017 N/A N/A 8811482727 4508,60000 P.O:NAMATI P.S:NAZIRA PIN:785685 DIST:SIVASAGAR 7. BINOD KUMAR RAM CHARITRA VILL:L.K ROAD MOBC 2017 N/A N/A 9954956743 4510,60000 MANDAL MANDAL GANDHINAGAR P.O:HAIBORGAON P.S:SADAR PIN:782002 DIST:NAGAON Students details 1st year 8. MANDIRA BORAH AKAN CH. BORAH VILL:BHALBHALIA GAON GEN. 2017 N/A N/A 7086270234 4511,60000 P.O:KAMPUR P.S:KAMPUR PIN:782426 DIST:NAGAON 9. -
Selected List
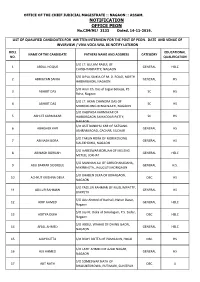
OFFICE OF THE CHIEF JUDICIAL MAGISTRATE :: NAGAON :: ASSAM. NOTIFICATION OFFICE PEON No.CJM(N)/ 3133 Dated, 14-11-2019. LIST OF QUALIFIED CANDIDATES FOR WRITTEN INTERVIEW FOR THE POST OF PEON. DATE AND VENUE OF INVERVIEW / VIVA VOCA WILL BE NOTIFY LATERON ROLL EDUCATIONAL NAME OF THE CANDIDATE FATHERS NAME AND ADDRESS CATEGORY NO. QUALIFICATION S/O LT. GULAM RASUL OF 1 ABDUL HOQUE GENERAL HSLC CHRISHTANPATTY, NAGAON S/O BIPUL SAIKIA OF M. D. ROAD, NORTH 2 ABHIGYAN SAIKIA GENERAL HS HAIBARGAON, NAGAON S/O Arun Ch. Das of Jagial Bebejia, PS. 3 ABHIJIT DAS SC HS Raha, Nagaon S/O LT. AKAN CHANDRA DAS OF 4 ABHIJIT DAS SC HS MORIKOLONG BENGENAATI, NAGAON S/O KALIPADA KARMAKAR OF 5 ABHIJIT KARMAKAR HAIBORGAON SAMADDAR PATTY, SC HS NAGAON S/O ASIT BANDHU KAR OF SATSANG 6 ABHISHEK KAR GENERAL HS ASHRAM ROAD, CACHAR, SILCHAR S/O TARUN BORA OF MORIKOLONG 7 ABINASH BORA GENERAL HS SIALEKHOWA, NAGAON S/O HARESWAR BORUAH OF MELENG 8 ABINASH BORUAH GENERAL HSLC METELI, JORHAT S/O MANNAN ALI OF SARUCHAKADAHA, 9 ABU BAKKAR SIDDIQUE GENERAL H.S. MIKIRBHETA, JALUGUTI,MORIGAON S/O BHABEN DEKA OF BORAGAON, 10 ACHYUT KRISHNA DEKA OBC HS NAGAON S/O FAZILUR RAHMAN OF MUSLIMPATTY, 11 ADILUR RAHMAN GENERAL HS BARPETA S/O Aziz Ahmed of Itachali, Natun Bazar, 12 ADIP AHMED GENERAL HSLC Nagaon S/O Joy Kt. Deka of Simaluguri, P.S. Sadar, 13 ADITYA DEKA OBC HSLC Nagaon S/O ABDUL WAHAB OF DHING GAON, 14 AFJAL AHMED GENERAL HSLC NAGAON 15 AJAY DUTTA S/O BIJOY DUTTA OF PAMGAON, HOJAI OBC HS S/O LATIF AHMED OF AZAD NAGAR, 16 AJIJ AHMED GENERAL HS NAGAON S/O SOMESWAR NATH OF 17 AJIT NATH OBC X BHALUKEKHOWA, PUTIMARI, SUNITPUR S/O TITARAM PANGGING OF SONARI, 18 AJOY PANGGING STP HS CHARAIDEO S/O LT AKON BORAH OF GHAGRAPARA 19 AKANTA BORAH OBC HS CHUBURI, SONITPUR S/O LT PRAFULLA LASKAR OF BARKOLA, 20 AKASH LASKAR OBC HSLC NAGAON S/O Girish Raja of Pub Solmara, P.S. -

Information Regarding Representatives of Pris (17 Nos of ZPC Under Golaghat Zila Parisahd.) Name of Pris Previous Sl.No
Information Regarding Representatives of PRIs (17 Nos of ZPC under Golaghat Zila Parisahd.) Name of PRIs Previous Sl.No. Name of Dist Name of Representatives Degn & Address Sex Caste Education Total No. Party Contact No (ZPC) Experiences 1 Athgaon Smti Urmila Saikia Member F G INC 9435152501 2 Bokakhat Smti Hemkumari Das Member F SC INC 9435510098 3 Borpathar Smti Ranjumoni Chetia Member F OBC INC 7399431763 4 Brahmaputra Sri Roten Kr. Das Member M SC INC 7896898626 5 Dakhinhengera Md. Mosim Hussain Member M G INC 6 Dergaon Sri Bhoben Gogoi Member M OBC INC 9707604573 7 Doyang Smti Lalita Gogoi Sonowal Member F ST INC 9954543099 8 Kathalguri Smti Momota Bora Member F OBC INC 9854808482 9 Kaziranga Sri Dharmeswar Kurmi Member M TGL INC 9829992040 Golaghat 10 Khumtai Smti Hiranyamoyee Bora President,Golaghat Zila Parishad F OBC INC 9401188087 11 Mohura Sri Chitra Raj Borboruah Member M OBC INC 9954973303 12 Morongi Smti Roseline Tirkey Member F TGL INC 9401126475 13 Navajyoti Sri Suresh Kr. Bodo Member M ST INC 9859930227 14 Oating Smti Rupa Saikia Member F G INC 9854824021 15 Ratanpur Sri Ranjit Musahari Member M ST INC 9859656407 Vice-President,Golaghat Zila 16 Rongamati Smti Ripamoni Doley Pegu F ST INC 8399007566 Parisahd 17 Sarupathar Smti Jeuti Nag Kullu Member F TGL INC Chief Executive Officer Golaghat Zila Parishad Information Regarding Representatives of PRIs ( 8Nos. Of APs under Golaghat Zila Parishad) Previous Name Name of PRIs Degn & Address Educati Total Sl.No. Name of Representatives Sex Caste Experienc Party Contact No of Dist (Aps) on No. -

People of the Margins Philippe Ramirez
People of the Margins Philippe Ramirez To cite this version: Philippe Ramirez. People of the Margins. Spectrum, 2014, 978-81-8344-063-9. hal-01446144 HAL Id: hal-01446144 https://hal.archives-ouvertes.fr/hal-01446144 Submitted on 25 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. People of the Margins People of the Margins Across Ethnic Boundaries in North-East India Philippe Ramirez SPECTRUM PUBLICATIONS GUWAHATI : DELHI In association with CNRS, France SPECTRUM PUBLICATIONS • Hem Barua Road, Pan Bazar, GUWAHATI-781001, Assam, India. Fax/Tel +91 361 2638434 Email [email protected] • 298-B Tagore Park Extn., Model Town-1, DELHI-110009, India. Tel +91 9435048891 Email [email protected] Website: www.spectrumpublications.in First published in 2014 © Author Published by arrangement with the author for worldwide sale. Unless otherwise stated, all photographs and maps are by the author. All rights reserved. Except for brief quotations in critical articles or reviews, no part of this publication may be reproduced, stored in a retrieval system, or transmitted/used in any form by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the publishers.