United States Patent Office Patented Apr
Total Page:16
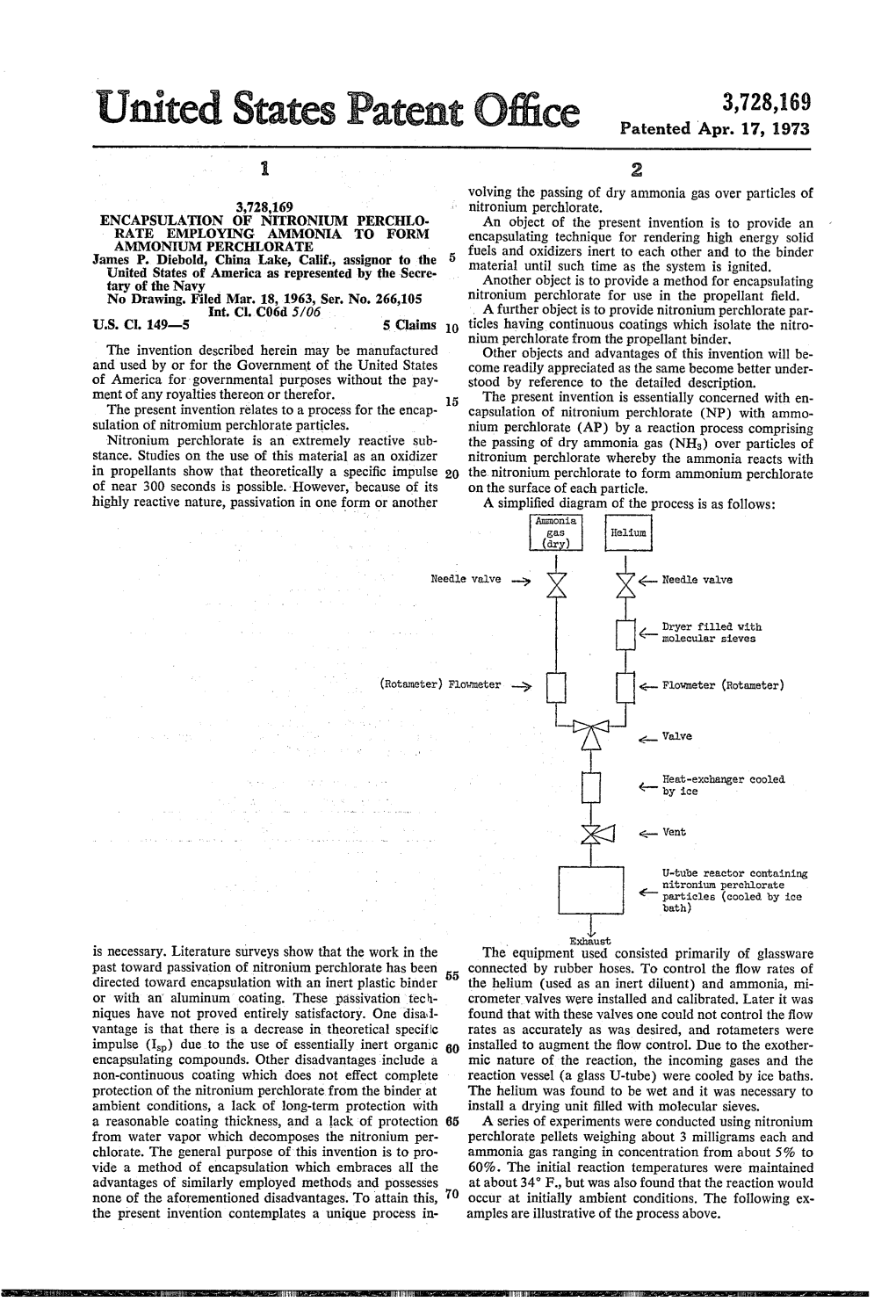
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON FACULTY OF SCIENCE DEPARTMENT OF CHEMISTRY Doctor of Philosophy CHEMICAL AND ELECTROCHEMICAL NITRATIONS OF ALKENES AND DIENES by Andrew Jonathan Bloom ACKNOWLEDGEMENT I wish to thank the following: My parents for their support; Dr. John Mellor and Professor Martin Fleischmann, my supervisors; Dr. Philip Parsons for his faith and encouragement; Neil Smith, Ivan Pinto, Jeff Buchannan and Neil Carlson for the proof-reading and Suzanne Salt for the typing. Financial support from The Procurement Executive, Ministry of Defence, for my maintenance grant, and NATO, for travel expenses, is gratefully acknowledged. Table of Contents Chapter 1 Introduction 1 I'l Electrochemical -

CHEMICAL HYGIENE PLAN Laboratory Safety Manual Approval
CHEMICAL HYGIENE PLAN Laboratory Safety Manual Approval Date: March 10, 2015 Prepared By: Environmental Health & Safety Table of Contents TABLE OF CONTENTS PART A: University-wide Policies and Procedures I. Introduction: ............................................................................... 2 II. Responsibilities: ......................................................................... 3 III. Information Dissemination and Employee Training. ................. 5 IV. Chemical Procurement Procedures. ........................................... 6 V. Working with Chemicals: General Safety Standard Operating Procedures. .................... 7 VI. Chemical Storage. ..................................................................... 9 VII. Disposal of Chemical Waste. ........................................................ 17 VIII. Hazard Identification: Classification & Communication. ........... 25 IX. Environmental Monitoring. ......................................................... 35 X. Personal Protective Equipment. .................................................. 37 XI. Respiratory Protection. ................................................................ 39 XII. Eye-Wash & Deluge Shower Stations. ......................................... 40 XIII. Fume Hoods. ............................................................................... 44 XIV. Fire Extinguishers. ..................................................................... 42 XV. Emergency Response. ................................................................. -

Section VIII Chemical Storage
Section VIII Chemical Storage General Considerations for Chemical Storage Carefully read the label before storing a hazardous chemical. The MSDS will also provide any special storage information and incompatibilities. Do Not Store Chemicals Alphabetically, except within a hazard class. Hazard classes that should be stored separately include: radioactive materials pyrophoric materials flammable materials oxidizing materials water reactive substances oxidizing acids inorganic acids organic acids caustics (bases) poisons (general laboratory reagents separated into organic and inorganic groups) Provide physical segregation (sills, curbs, trays) or separation between hazard classes. Keep flammable materials by themselves in approved storage cans, cabinets, or rooms. Store oxidizers well away from flammable materials. Please refer to Appendix E for an example Chemical Compatibility Chart Segregation Do not store unsegregated chemicals in alphabetical order or incompatible chemicals in close proximity to each other. The amount of space that can be placed between different chemical classes depends on the amount of storage area available in the lab suite. Do not segregate chemical classes into separate rooms unless they will only be used in that room. Segregation that disrupts normal work flow or requires more frequent transport of chemicals between labs will increase the probability of a chemical spill. Use common sense in planning chemical storage areas. Store dry reagents, liquids reagents and solutions and compressed gases in separate areas. Within each of these chemical forms segregate into hazard classes. Segregate dry reagents as follows: oxidizing solids flammable solids water reactive solids all others solids Segregate liquid reagents and solutions as follows: acid liquids caustic liquids oxidizing liquids perchloric acid solutions flammable or combustible liquids all other liquids Segregate compressed gases as follows: toxic gases flammable gases oxidizing and inert gases Once separated into hazard classes, chemicals may be stored alphabetically. -

Commerce in Explosives; 2020 Annual Those on the Annual List
Federal Register / Vol. 85, No. 247 / Wednesday, December 23, 2020 / Notices 83999 inspection at the Office of the Secretary or synonyms in brackets. This list Black powder substitutes. and on EDIS.3 supersedes the List of Explosive *Blasting agents, nitro-carbo-nitrates, This action is taken under the Materials published in the Federal including non-cap sensitive slurry and authority of section 337 of the Tariff Act Register on January 2, 2020 (Docket No. water gel explosives. of 1930, as amended (19 U.S.C. 1337), 2019R–04, 85 FR 128). Blasting caps. and of §§ 201.10 and 210.8(c) of the The 2020 List of Explosive Materials Blasting gelatin. Commission’s Rules of Practice and is a comprehensive list, but is not all- Blasting powder. Procedure (19 CFR 201.10, 210.8(c)). inclusive. The definition of ‘‘explosive BTNEC [bis (trinitroethyl) carbonate]. materials’’ includes ‘‘[e]xplosives, BTNEN [bis (trinitroethyl) nitramine]. By order of the Commission. BTTN [1,2,4 butanetriol trinitrate]. Issued: December 18, 2020. blasting agents, water gels and detonators. Explosive materials, Bulk salutes. William Bishop, include, but are not limited to, all items Butyl tetryl. Supervisory Hearings and Information in the ‘List of Explosive Materials’ Officer. C provided for in § 555.23.’’ 27 CFR Calcium nitrate explosive mixture. [FR Doc. 2020–28458 Filed 12–22–20; 8:45 am] 555.11. Accordingly, the fact that an BILLING CODE 7020–02–P Cellulose hexanitrate explosive explosive material is not on the annual mixture. list does not mean that it is not within Chlorate explosive mixtures. coverage of the law if it otherwise meets DEPARTMENT OF JUSTICE Composition A and variations. -

Chemical Hygiene Plan
Chemical Hygiene Plan Prepared by Lori Jennis, CIH of ECOS Inc. under direction of: Stephen D. Kucera, Ph.D. Chemical Hygiene & Biological Safety Officer The University of Tampa 401 W. Kennedy Blvd Tampa, FL 33606-1490 January 2021 Edition Version 1.3 Chemical Hygiene Plan for The University of Tampa Effective January 2021 TABLE OF CONTENTS 1.0 Introduction ............................................................................................................. 1 1.1. Purpose ................................................................................................................ 1 1.2. Intent .................................................................................................................... 1 1.3. Organization ........................................................................................................ 1 1.4. Location ............................................................................................................... 2 1.5. Chemical Safety Website .................................................................................... 2 2.0 Responsibilities ........................................................................................................ 2 2.1. President of the University .................................................................................. 2 2.2. Chemical Hygiene & Biological Safety Officer .................................................. 3 2.3. Principal Investigator.......................................................................................... -

Summary of Chemical Hazards
CHEMICAL HAZARDS Taken in part from: www.southalabama.edu/environmental/ chemicalhygieneplanappendixb2003.DOC Chemicals are considered hazardous if they pose either PHYSICAL or HEALTH hazards to workers exposed to them. PHYSICAL HAZARDS include: • Fire or explosions • Sudden releases of pressure (for example what happens when a tank of compressed gas is punctured) and: • Reactivity (if a chemical can burn, explode or release dangerous gases after contact with water, air or other chemicals). HEALTH HAZARDS are illnesses or other health problems that could develop as a result of exposure to a hazardous chemical. Health hazards could be as minor as a headache or mild skin irritation or as major as cancer (or in rare cases, death) HAZARD TYPES CORROSIVE—substances that by direct chemical action are injurious to body tissue or corrosive to metal. Corrosive injury may be to a minor degree (irritation) or actual physical disruption of the body tissues. COMMON CORROSIVE LIQUIDS INORGANIC ACIDS ORGANIC ACIDS OTHER INORGANICS Chlorosulfonic Acetic Bromine Chromic Butyric Phosphorous Trichloride Hydrochloric Chloroacetic Silicon Tetrachloride Nitric Formic Sulfuryl Chloride Sulfuric Thionyl Chloride Peroxides CAUSTIC SOLUTIONS ORGANIC SOLVENTS OTHER ORGANICS Ammonia Dichlorethylene Acetic Anhydride Sodium Hydroxide Ethylene Chlorohydrin Liquefied Phenol Potassium Hydroxide Perchloroethylene Triethanolamine Methyl Ethyl Ketone 2-Aminoethanol Gasoline HAZARDS AND PRECAUTIONARY MEASURES Everyone knows mineral acids can cause burns. Few persons realize the extent to which they can damage body tissue. The concentration and duration of exposure control the degree of injury. The primary modes of attack of corrosive liquids are the skin and eyes. Concentrated alkaline solutions have a more corrosive effect on tissue than most strong acids. -

Interim Drinking Water Health Advisory for Perchlorate
Interim Drinking Water Health Advisory For Perchlorate ii Interim Drinking Water Health Advisory for Perchlorate Prepared by: Health and Ecological Criteria Division Office of Science and Technology Office of Water U.S. Environmental Protection Agency Washington, DC 20460 http://www.epa.gov/waterscience/ EPA 822-R-08-025 December 2008 iii iv TABLE OF CONTENTS ACKNOWLEDGMENTS........................................................................................................................................VI LIST OF ABBREVIATIONS ................................................................................................................................ VII 1.0 INTRODUCTION .......................................................................................................................................... 1 2.0 GENERAL INFORMATION AND PROPERTIES.................................................................................... 2 2.1 PHYSICAL AND CHEMICAL PROPERTIES ..................................................................................................... 2 2.2 USES.............................................................................................................................................................. 3 3.0 OCCURRENCE AND EXPOSURE ............................................................................................................. 3 3.1 AIR ............................................................................................................................................................... -
Faculty Handbook
APPENDIX 1 Safety or Personal Protective Equipment This policy is for all faculty and staff and can be found on the Human Resources web pages at: http://www.rochester.edu/working/hr/policies/pdfpolicies/158.pdf . APPENDIX 2 Reproductive Protection Policy This policy is for all faculty and staff and can be found on the Human Resources Web pages at: http://www.rochester.edu/working/hr/policies/pdfpolicies/167.pdf . APPENDIX 3 OSHA Laboratory Safety Standard The OSHA Laboratory Safety Standard is available at the following web site: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10106 APPENDIX 4 Research & Clinical Laboratory Waste Disposal Please see the Environmental Health & Safety Web Site: http://www.safety.rochester.edu/restricted/labwastetable.pdf APPENDIX 5 Request for SDS DATE:____/_____/______ NAME:____________________________ DEPARTMENT:__________________________ ROOM#:__________________ P.O. BOX:_________________ PHONE:______________ EH&S recommends SDSs for high hazard chemicals be retained in the laboratory. If you do not have a current copy of these safety sheets, they are available through the internet. For any SDSs you were not able to obtain through the internet, complete the requested information below. Print the complete name of the chemical. Providing the information in columns 2 and 3 can reduce the time required to obtain the SDS. CHEMICAL NAME MANUFACTURER & CAT # ADDRESS Return form via Fax to: 274-0001 QUESTIONS? Call x5-3241 or Return form via intradepartmental mail to: EH&S, Box 278878 APPENDIX 6 Peroxidizable Compounds Organic peroxides are considered low-power explosives that are sensitive to shock, sparks, and other accidental ignition. -

Revised November 2019
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ EMERGENCY TELEPHONE NUMBERS: 911 for Emergency and urgent consultation 3-2035 MSU Police; 3-2066 Facilities Management 3- 2915 Dr. Mark Blankenbuehler, Associate Professor Chemistry & Chemical Safety Committee Chair; Office: 404D Lappin Hall; email: [email protected] 3-2179 or (606) 207-9425 Holly Niehoff, Director of EHS, Risk Management & Insurance; Office BCB 113; email: [email protected] 3-9022 or (606) 207-2556 Derek Lewis, Fire & Life Safety Technician; Office BCB 113; email: [email protected] 3-______ Department Chair 3-______ Building Supervisor State 24-hour warning point for HAZMAT Spill Notification If you need to report a spill in accordance with SARA Title III Section 304 and KRS 39E.190, please contact the Duty Officer at the Commonwealth Emergency Operations Center at 800.255.2587 which serves as the twenty-four (24) hour warning point and contact for the Commonwealth Emergency Response Commission. Revised November 2019 All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboratory Standard 29 CFR 19190.1450. Morehead State University laboratories may use this document as a starting point for creating their work area specific Chemical Hygiene Plan (CHP). Morehead State Chemical Hygiene Plan MSU Laboratory Awareness Certification For CHP of (Professor, Building, Room)________________________ The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). -

Emergency Phone Numbers
HAZARDOUS MATERIALS SAFETY MANUAL ENVIRONMENTAL HEALTH & SAFETY Emergency Phone Numbers Newark Campus: Fire ..........................................................911 University Police ....................................911 Ambulance .............................................911 Student Health Center ............................2226 Lewes Campus Fire ..........................................................9-911 Police Emergency ....................................9-911 Ambulance ..............................................9-911 University Police .....................................4333 Georgetown Campus Fire ..........................................................9-911 Police Emergency ....................................9-911 Ambulance ..............................................9-911 Wilmington Campus Fire ..........................................................9-911 Police Emergency ....................................9-911 Ambulance ..............................................9-911 University Police .....................................2222 Dover Campus Fire ..........................................................99-911 Police Emergency ....................................99-911 Ambulance ..............................................99-911 Poison Information Center Local ..........................................1-800-722-7112 National .....................................1-800-222-1222 July 2007 Environmental Health and Safety General Services Bldg., Room 132 222 S. Chapel Street Newark, DE 19716 (302) 831-8475 -

United States Patent Office
3,137,599 United States Patent Office Patented June 16, 1964 2 3,137,599 unsaturated hydrocarbon polymer in the presence of a POLYSLANE ROCKET PROPELLANTS free radical generator such as organic peroxides or under Richard W. Aisgaard, Henry N. Beck, and Edwin P. the influence of ionizing radiation or in the presence of Plueddemann, Midland, Mich., assigors to Dow a platinum catalyst such as chloroplatinic acid or platinum Corning Corporation, Midland, Mich., a corporation of dispersed on charcoal. Any of these catalysts will, upon Michigan the application of heat, cause addition of the SiH groups No Drawing. Fied Oct. 25, 1960, Ser. No. 64,953 to the C=C bond, thereby inducing gelation of the mix 2 Claims. (C. 149-19) ture and bonding the entire mass, including the oxidizer, into a unitary whole. This invention relates to the use of polysilanes as fuels 10 It should be understood, of course, that the silane alone in rocket propellants. can serve as the bonding agent for the oxidizer. This invention particularly relates to the art of solid It should be understood that the silanes of this inven propellants. It has long been known that with the present tion can be used to either improve the performance of ly known rocket propellants liquid fuels deliver more presently employed solid fuels, such as organic resin thrust per pound of fuel than solid propellants. On the 5 polymers, either alone or in admixture with oxidizable other hand, it is well known that solid fuels require a metals, such as aluminum, or the silanes can be used simpler vehicle than do the liquid fuels.