Chemical Hygiene Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON FACULTY OF SCIENCE DEPARTMENT OF CHEMISTRY Doctor of Philosophy CHEMICAL AND ELECTROCHEMICAL NITRATIONS OF ALKENES AND DIENES by Andrew Jonathan Bloom ACKNOWLEDGEMENT I wish to thank the following: My parents for their support; Dr. John Mellor and Professor Martin Fleischmann, my supervisors; Dr. Philip Parsons for his faith and encouragement; Neil Smith, Ivan Pinto, Jeff Buchannan and Neil Carlson for the proof-reading and Suzanne Salt for the typing. Financial support from The Procurement Executive, Ministry of Defence, for my maintenance grant, and NATO, for travel expenses, is gratefully acknowledged. Table of Contents Chapter 1 Introduction 1 I'l Electrochemical -

CHEMICAL HYGIENE PLAN Laboratory Safety Manual Approval
CHEMICAL HYGIENE PLAN Laboratory Safety Manual Approval Date: March 10, 2015 Prepared By: Environmental Health & Safety Table of Contents TABLE OF CONTENTS PART A: University-wide Policies and Procedures I. Introduction: ............................................................................... 2 II. Responsibilities: ......................................................................... 3 III. Information Dissemination and Employee Training. ................. 5 IV. Chemical Procurement Procedures. ........................................... 6 V. Working with Chemicals: General Safety Standard Operating Procedures. .................... 7 VI. Chemical Storage. ..................................................................... 9 VII. Disposal of Chemical Waste. ........................................................ 17 VIII. Hazard Identification: Classification & Communication. ........... 25 IX. Environmental Monitoring. ......................................................... 35 X. Personal Protective Equipment. .................................................. 37 XI. Respiratory Protection. ................................................................ 39 XII. Eye-Wash & Deluge Shower Stations. ......................................... 40 XIII. Fume Hoods. ............................................................................... 44 XIV. Fire Extinguishers. ..................................................................... 42 XV. Emergency Response. ................................................................. -

Study on the Formation and the Decomposition of Agn3 and A
Study on the formation and the decomposition of AgN3 and a hypothetical compound ReN3 by using density functional calculations. G. Soto. Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología Km 107 carretera Tijuana-Ensenada, Ensenada Baja California, México. Corresponding Author: G. Soto. CNyN-UNAM P.O. Box 439036, San Ysidro, CA 92143-9036, USA Tel: +52+646+1744602, Fax: +52+646+1744603 E-Mail: [email protected] Abstract We present a comparative study between ReN3 and AgN3 by using density functional theory. The ReN3 is a hypothetical compound proposed by us to interpret the Re to Re interplanar spacing of thin films grown by sputtering. Both, the AgN3 as the ReN3, are calculated as positive enthalpy compounds. The enthalpy might give a clue about the spontaneous decomposition of the solid form, but it cannot be interpreted as a restriction of its synthesizability. As from the calculated total-energy, we discuss the route for the formation of AgN3 starting from atomic species in aqueous solution. We propose that their synthesizability is conditioned by the energy of free nitrogen atoms, and the kinetics of reaction. We conclude that the intrinsic stability of a certain atomic arrangement depends only of the equilibrium of atomic forces, and not from the energy value associated with that structure. 1 1. Introduction Predicting new solids based solely on computer calculations is one of the main challenges of materials science. Achieving this would mean a giant step forward as it would save many hours of fruitless efforts. Although there has been significant progress[1], it is still early to sing praises. -

Section VIII Chemical Storage
Section VIII Chemical Storage General Considerations for Chemical Storage Carefully read the label before storing a hazardous chemical. The MSDS will also provide any special storage information and incompatibilities. Do Not Store Chemicals Alphabetically, except within a hazard class. Hazard classes that should be stored separately include: radioactive materials pyrophoric materials flammable materials oxidizing materials water reactive substances oxidizing acids inorganic acids organic acids caustics (bases) poisons (general laboratory reagents separated into organic and inorganic groups) Provide physical segregation (sills, curbs, trays) or separation between hazard classes. Keep flammable materials by themselves in approved storage cans, cabinets, or rooms. Store oxidizers well away from flammable materials. Please refer to Appendix E for an example Chemical Compatibility Chart Segregation Do not store unsegregated chemicals in alphabetical order or incompatible chemicals in close proximity to each other. The amount of space that can be placed between different chemical classes depends on the amount of storage area available in the lab suite. Do not segregate chemical classes into separate rooms unless they will only be used in that room. Segregation that disrupts normal work flow or requires more frequent transport of chemicals between labs will increase the probability of a chemical spill. Use common sense in planning chemical storage areas. Store dry reagents, liquids reagents and solutions and compressed gases in separate areas. Within each of these chemical forms segregate into hazard classes. Segregate dry reagents as follows: oxidizing solids flammable solids water reactive solids all others solids Segregate liquid reagents and solutions as follows: acid liquids caustic liquids oxidizing liquids perchloric acid solutions flammable or combustible liquids all other liquids Segregate compressed gases as follows: toxic gases flammable gases oxidizing and inert gases Once separated into hazard classes, chemicals may be stored alphabetically. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Commerce in Explosives; 2020 Annual Those on the Annual List
Federal Register / Vol. 85, No. 247 / Wednesday, December 23, 2020 / Notices 83999 inspection at the Office of the Secretary or synonyms in brackets. This list Black powder substitutes. and on EDIS.3 supersedes the List of Explosive *Blasting agents, nitro-carbo-nitrates, This action is taken under the Materials published in the Federal including non-cap sensitive slurry and authority of section 337 of the Tariff Act Register on January 2, 2020 (Docket No. water gel explosives. of 1930, as amended (19 U.S.C. 1337), 2019R–04, 85 FR 128). Blasting caps. and of §§ 201.10 and 210.8(c) of the The 2020 List of Explosive Materials Blasting gelatin. Commission’s Rules of Practice and is a comprehensive list, but is not all- Blasting powder. Procedure (19 CFR 201.10, 210.8(c)). inclusive. The definition of ‘‘explosive BTNEC [bis (trinitroethyl) carbonate]. materials’’ includes ‘‘[e]xplosives, BTNEN [bis (trinitroethyl) nitramine]. By order of the Commission. BTTN [1,2,4 butanetriol trinitrate]. Issued: December 18, 2020. blasting agents, water gels and detonators. Explosive materials, Bulk salutes. William Bishop, include, but are not limited to, all items Butyl tetryl. Supervisory Hearings and Information in the ‘List of Explosive Materials’ Officer. C provided for in § 555.23.’’ 27 CFR Calcium nitrate explosive mixture. [FR Doc. 2020–28458 Filed 12–22–20; 8:45 am] 555.11. Accordingly, the fact that an BILLING CODE 7020–02–P Cellulose hexanitrate explosive explosive material is not on the annual mixture. list does not mean that it is not within Chlorate explosive mixtures. coverage of the law if it otherwise meets DEPARTMENT OF JUSTICE Composition A and variations. -

Chemical Hygiene Plan
Chemical Hygiene Plan Prepared by Lori Jennis, CIH of ECOS Inc. under direction of: Stephen D. Kucera, Ph.D. Chemical Hygiene & Biological Safety Officer The University of Tampa 401 W. Kennedy Blvd Tampa, FL 33606-1490 January 2021 Edition Version 1.3 Chemical Hygiene Plan for The University of Tampa Effective January 2021 TABLE OF CONTENTS 1.0 Introduction ............................................................................................................. 1 1.1. Purpose ................................................................................................................ 1 1.2. Intent .................................................................................................................... 1 1.3. Organization ........................................................................................................ 1 1.4. Location ............................................................................................................... 2 1.5. Chemical Safety Website .................................................................................... 2 2.0 Responsibilities ........................................................................................................ 2 2.1. President of the University .................................................................................. 2 2.2. Chemical Hygiene & Biological Safety Officer .................................................. 3 2.3. Principal Investigator.......................................................................................... -

Summary of Chemical Hazards
CHEMICAL HAZARDS Taken in part from: www.southalabama.edu/environmental/ chemicalhygieneplanappendixb2003.DOC Chemicals are considered hazardous if they pose either PHYSICAL or HEALTH hazards to workers exposed to them. PHYSICAL HAZARDS include: • Fire or explosions • Sudden releases of pressure (for example what happens when a tank of compressed gas is punctured) and: • Reactivity (if a chemical can burn, explode or release dangerous gases after contact with water, air or other chemicals). HEALTH HAZARDS are illnesses or other health problems that could develop as a result of exposure to a hazardous chemical. Health hazards could be as minor as a headache or mild skin irritation or as major as cancer (or in rare cases, death) HAZARD TYPES CORROSIVE—substances that by direct chemical action are injurious to body tissue or corrosive to metal. Corrosive injury may be to a minor degree (irritation) or actual physical disruption of the body tissues. COMMON CORROSIVE LIQUIDS INORGANIC ACIDS ORGANIC ACIDS OTHER INORGANICS Chlorosulfonic Acetic Bromine Chromic Butyric Phosphorous Trichloride Hydrochloric Chloroacetic Silicon Tetrachloride Nitric Formic Sulfuryl Chloride Sulfuric Thionyl Chloride Peroxides CAUSTIC SOLUTIONS ORGANIC SOLVENTS OTHER ORGANICS Ammonia Dichlorethylene Acetic Anhydride Sodium Hydroxide Ethylene Chlorohydrin Liquefied Phenol Potassium Hydroxide Perchloroethylene Triethanolamine Methyl Ethyl Ketone 2-Aminoethanol Gasoline HAZARDS AND PRECAUTIONARY MEASURES Everyone knows mineral acids can cause burns. Few persons realize the extent to which they can damage body tissue. The concentration and duration of exposure control the degree of injury. The primary modes of attack of corrosive liquids are the skin and eyes. Concentrated alkaline solutions have a more corrosive effect on tissue than most strong acids. -

Interim Drinking Water Health Advisory for Perchlorate
Interim Drinking Water Health Advisory For Perchlorate ii Interim Drinking Water Health Advisory for Perchlorate Prepared by: Health and Ecological Criteria Division Office of Science and Technology Office of Water U.S. Environmental Protection Agency Washington, DC 20460 http://www.epa.gov/waterscience/ EPA 822-R-08-025 December 2008 iii iv TABLE OF CONTENTS ACKNOWLEDGMENTS........................................................................................................................................VI LIST OF ABBREVIATIONS ................................................................................................................................ VII 1.0 INTRODUCTION .......................................................................................................................................... 1 2.0 GENERAL INFORMATION AND PROPERTIES.................................................................................... 2 2.1 PHYSICAL AND CHEMICAL PROPERTIES ..................................................................................................... 2 2.2 USES.............................................................................................................................................................. 3 3.0 OCCURRENCE AND EXPOSURE ............................................................................................................. 3 3.1 AIR ............................................................................................................................................................... -
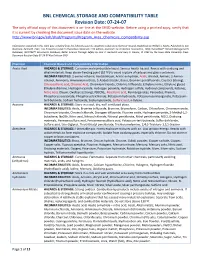
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title DETERMINATION OF AZIDE ION BY HYDROGEN ION TITRATION AFTER OXIDATION WITH NITRITE Permalink https://escholarship.org/uc/item/39q6h8sg Authors Clem, Ray G. Huffman, E.H. Publication Date 1964-11-01 eScholarship.org Powered by the California Digital Library University of California UCRL-11777 University of California Ernest 0. lawrence Radiation Laboratory DETERMINATION OF AZIDE ION BY HYDROGEN ION TITRATION AFTER OXIDATION WITH NITRITE TWO-WEEK lOAN COPY This is a library Circulating Copy which may be borrowed for two weeks. For a personal retention copy, call Tech. Info. Division, Ext. 5545 Berkeley, California DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of Califomia. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Govemment or any agency thereof or the Regents of the University of California.