Mayeen the Presence of Toxic Metals.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abundance and Biodiversity of Phytoplankton in the Halda River During Monsoon and Post Monsoon Period
Int. J. Adv. Res. Biol. Sci. (2021). 8(8): 10-18 International Journal of Advanced Research in Biological Sciences ISSN: 2348-8069 www.ijarbs.com DOI: 10.22192/ijarbs Coden: IJARQG (USA) Volume 8, Issue 8 -2021 Research Article DOI: http://dx.doi.org/10.22192/ijarbs.2021.08.08.002 Abundance and Biodiversity of Phytoplankton in the Halda River during Monsoon and Post Monsoon period. Mazharul Islam Sajeeb Institute of Marine Sciences, University of Chittagong, Bangladesh. E-mail: [email protected] Abstract Abundance of phytoplankton in the Halda river was studied during monsoon (July) & post monsoon (February). Samples were collected from three sampling stations- Station 01(Moduna Ghat), Station 02 (Sattar Ghat), Station 03 (Najirbat). Total 20 species were identified. The highest number were recorded for Coscinodiscus Sp (600 cells/liter) during post monsoon at station 03. The average number of phytoplankton in station 01, station 02 and station 03 were found 2985 cells/liter, 3470 cells/liter, 3870 cells/liter. The most abundance of phytoplankton was found in post monsoon. The physio chemical parameter varied from as, temperature 27°to 29° during monsoon , 25°to 28° during post monsoon, PH 5.5 to 6 during monsoon and 5.5 to 5.9 during post monsoon, dissolve oxygen 4.285 mg/l to 5mg/l during monsoon, 4.285mg/l to 5.71 mg/l during post monsoon and transparency 26.5 to 32 cm during monsoon, 27 to 31 cm during post monsoon. The Shannon diversity index was ranged between 2.345 to 2.6 during monsoon and 2.59 to 2.62 during post monsoon. -

Risk and Coping Mechanisms of the Carp Spawn Fishing Community of the Halda River, Bangladesh
Bangladesh J. Zool. 45(1): 85-96, 2017 ISSN: 0304-9027 (print) 2408-8455 (online) RISK AND COPING MECHANISMS OF THE CARP SPAWN FISHING COMMUNITY OF THE HALDA RIVER, BANGLADESH Aysha Akhtar, Md. Tarikul Islam, Md. Shafiqul Islam, Muhammad Moznu Mia, Md. Simul Bhuyan*, Md. Manzoorul Kibria1, Abu Sayeed Muhammad Sharif2, and Abu Hena Mustafa Kamal3 Institute of Marine Sciences and Fisheries, University of Chittagong, Chittagong, Bangladesh Abstract: To assess the livelihood risks and coping mechanisms of the spawn fishing community of the Halda river, Chittagong, Bangladesh was conducted. Four areas, namely Ankurighona, Gorduara, Madarimukh and Madunaghat were selected on the basis of the aggregation of spawn fishers. A structured questionnaire survey was made on 152 spawn fishers in two categories i.e. boat owner and hired laborers to collect primary data. Three major man-made risks, namely catch of brood fish, non-functioning sluice gates and cutting of river bends related to egg collection were mentioned by the spawn fishers while salinity intrusion, river erosion and fluctuation in weather variables were found as the main natural risks. Willingness to continue egg collection despite the risks was disagreed by most of the respondents while those who were found to continue egg collection mentioned their driving forces as high profit, tradition and hobby. The respondents expressed that they cannot take any measures to mitigate the risks of salinity intrusion, weather fluctuation, mortality of spawn, non-functioning sluice gates, cutting of river bends and political influences. However, informing police and raising awareness to stop illegal catch of brood, construction of proper sluice gates, stop further cutting of river bends and rehabilitation of erosion victims were mentioned as probable solutions. -

The Case of Bangladesh D National Se
Globalization, Local Crimes and National Security: The Case of Bangladesh Submitted by: Md. Ruhul Amin Sarkar Session: 149/2014-2015 Department: International Relations University of Dhaka. P a g e | 1 Abstract Globalization has become one of the most significant phenomena in the world since the end of the cold war. Globalization especially the economic globalization has brought about new opportunities and opened dynamic windows for the people of the world based on the notion of liberalism, free market, easy access of goods and services. Although globalization has brought about some positive gains for individuals and society, it has caused negative impacts on the society called ‘the dark side of globalization’. It has created complex and multifaceted security problems and threats to the countries especially the developing countries like Bangladesh. Globalization has changed the nature and dynamics of crime although crime is not a new phenomenon in Bangladesh. The nature or pattern of crime has changed remarkably with the advent of globalization, modern technology and various modern devices, which pose serious security threats to the individuals, society and the country. Globalization has created easy access to conducting illegal trade such as small arms, illegal drugs and human trafficking and some violent activities such as kidnapping, theft, murder, around the world as well as in Bangladesh. It has developed the new trends of crimes, gun violence, drugs crime, and increasing number of juvenile convicts and heinous crimes committed in Bangladesh. Over the years, the number of organized murder crimes is increasing along with rape cases and pretty nature of crimes with the advent of globalization and information technology. -

Betbunia Chairy Bazar-Bara Aoulia- Santirhat Road District: Rangamati
Resettlement Plan Project No. 42248-013 Resettlement Plan May 2016 2763-BAN (SF): Second Chittagong Hill Tracts Rural Development Project Subproject: Betbunia Chairy Bazar-Bara Aoulia- Santirhat Road District: Rangamati Prepared by Chittagong Hill Tracts Regional Council for the People’s Republic of Bangladesh and the Asian Development Bank This resettlement plan is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. 'Feqls8i <l(stq.l T{sF "nfufi$q fr'$rs ra-fra.r "rftsf.fi -,r,fi {'\8Lfi qfi-{l{$. qTt n'i i:;.oo.oooo.ti..!.)8.scE.)A \r? e -f;1s )c/r,/t.,5r {il<a rr,io, Iosl{ G$,r sr,T *r 11fu e{ q+cq G15jGfi c5ift {]q]< {rd qrth$ rttttqEw*@'iAl F. l"i€r.€ ,!{ TtTs R-!?/)o!/"rtrfltq i/to),5/.eet Eiiilr, t8/q/t,,)s 'l].-o1 5Lrqtr "iai t*n :r+A 1l +ii:r e< Eril.n r-.tFilii (r.3] nqrn <ti qdl4rri .{k4 a[i -]qlr F qiiriIe.r € F,irrr.'rr Iiftrc- le- t.AllP 6- r'q6. a-({ qBi1n;r'r:d-r.T E...I t "G---.'f -*i,?l&{}-Y o,j aon rP:ln : bc8c.r1 ri,Jdheealion fri rahoo co l qofl {ioao ?ri'sI tucri 1A d:F]n err, qn "r{m q-or t.3<5t';ftT{. -

Physicochemical and Biological Monitoring of Water Quality of Halda River, Bangladesh
INTERNATIONAL JOURNAL OF ENVIRONMENTAL & SCIENCE EDUCATION e-ISSN: 1306-3065 OPEN ACCESS 2019, Vol. 14, No. 4, 169-181 Physicochemical and Biological Monitoring of Water Quality of Halda River, Bangladesh Mohammad Ayub Parvez 1, M. Main Uddin 2, Md. Kamrul Islam 1*, Md. Manzoorul Kibria 3 1 Research Associate, Institute of Forestry and Environmental Sciences University of Chittagong, Chittagong 4331, BANGLADESH 2 Assistant Professor, Institute of Forestry and Environmental Sciences University of Chittagong, Chittagong 4331, BANGLADESH 3 Professor, Department of Zoology, University of Chittagong, Chittagong 4331, BANGLADESH * CORRESPONDENCE: [email protected] ABSTRACT The tidal river Halda that serves as a natural breeding ground for major Indian carps and sources of other aquatic resources is of special interest. This study was conducted to monitor the water quality using physicochemical and biological parameters of the river in three different sampling stations namely Gorduara, Sattarghat and Kalurghat. Eight physicochemical parameters of water - temperature, PH, transparency, EC, DO, TDS, SS, salinity and plankton communities were considered for monitoring water quality in three stations. All the physicochemical parameters were within the pollution standard except DO (4.5 mgL-1) at Kalurghat station. In case of biological monitoring, zooplankton populations consisting of four classes were identified where 13 zooplankton genera under these 4 classes showing the dominancy. The abundance of zooplankton was higher at Gorduara station (2042 No./ L) followed by Sattarghat (1906 No./ L) and Kalurghat (1610 No./ L) respectively. On the basis of Identifying 11 genera of algal genus, six genera were used to prepare ‘Palmer pollution index’ which identified Kalurghat station as highly polluted zone. -

Department of Sociology University of Dhaka Dhaka University Institutional Repository
THE NATURE AND EXTENT OF HOMICIDE IN BANGLADESH: A CONTENT ANALYSIS ON REPORTS OF MURDER IN DAILY NEWSPAPERS T. M. Abdullah-Al-Fuad June 2016 Department of Sociology University of Dhaka Dhaka University Institutional Repository THE NATURE AND EXTENT OF HOMICIDE IN BANGLADESH: A CONTENT ANALYSIS ON REPORTS OF MURDER IN DAILY NEWSPAPERS T. M. Abdullah-Al-Fuad Reg no. 111 Session: 2011-2012 Submitted in partial fulfillment of the requirements of the degree of Master of Philosophy June 2016 Department of Sociology University of Dhaka Dhaka University Institutional Repository DEDICATION To my parents and sister Dhaka University Institutional Repository Abstract As homicide is one of the most comparable and accurate indicators for measuring violence, the aim of this study is to improve understanding of criminal violence by providing a wealth of information about where homicide occurs and what is the current nature and trend, what are the socio-demographic characteristics of homicide offender and its victim, about who is most at risk, why they are at risk, what are the relationship between victim and offender and exactly how their lives are taken from them. Additionally, homicide patterns over time shed light on regional differences, especially when looking at long-term trends. The connection between violence, security and development, within the broader context of the rule of law, is an important factor to be considered. Since its impact goes beyond the loss of human life and can create a climate of fear and uncertainty, intentional homicide (and violent crime) is a threat to the population. Homicide data can therefore play an important role in monitoring security and justice. -

Spatiotemporal Abundance and Distribution of Phytoplankton in the Halda River, Chittagong, Bangladesh Md
East African Scholars Journal of Agriculture and Life Sciences Abbreviated Key Title: East African Scholars J Agri Life Sci ISSN 2617-4472 (Print) | ISSN 2617-7277 (Online) | Published By East African Scholars Publisher, Kenya Volume-2 | Issue-6 | Jun-2019 | Research Article Spatiotemporal abundance and distribution of phytoplankton in the Halda River, Chittagong, Bangladesh Md. Mostafa Monwar1 and Foysal Alam Sharker1 1Institute of Marine Sciences, University of Chittagong, Chittagong 4331, Bangladesh *Corresponding Author Md. Mostafa Monwar Abstract: Seasonal variability of phytoplankton species composition, abundance and physic-chemical factors influencing phytoplankton dynamics were investigated in the Halda River, Chittagong, Bnagladesh. Samples were collected from three selected stations like Sattar ghat (Station 01), Moduna ghat (Station 02) and mouth of the Halda River near Kalurghat old bridge (Station 03) during monsoon and post monsoon. The average number of phytoplankton density were 8745 cells/litter, 10445 Cells/liter and 10715 cells/litter at station 01, station 02 and station 3 respectively. The phytoplankton abundance were higher in post monsoon (10853 cells/litter) compared to monsoon (9083 cells/litter). Total 20 genera were identified from the Halda River during this study. The highest count recorded for Coscinodiscus sp followed by Thalassiosira sp. Correlation of phytoplankton with some selected water parameters were also studied. Keywords: Phytoplankton, temporal and spatial distribution, Halda River, Bangladesh. INTRODUCTION Yamazi (1972) mentioned that phytoplankton The Halda River is one of the important river are the important source upon which all aquatic fauna of Chittagong district that fed by several hilly streams depends for the energy necessary for their existence. including 12 important tributaries (Azadi, 2004). -

Annual Human Rights Bulletin- Bangladesh Situation 2017
Annual Human Rights Bulletin Bangladesh Situation 2017 HUMAN RIGHTS SUPPORT SOCIETY [HRSS) www.hrssbd.org Annual Human Rights Bulletin Bangladesh Situation 2017 HUMAN RIGHTS SUPPORT SOCIETY (HRSS) www.hrssbd.org Annual Human Rights Bulletin Bangladesh Situation 2017 HRSS Any materials published in this Bulletin May be reproduced with acknowledgment of HRSS. Published by Human Rights Support Society 3D, 3rd Floor, Nurjehan Tower Outer Circular Road, Banglamotor Dhaka-1000, Bangladesh. Email: [email protected], [email protected] Website: www.hrssbd.org Cover & Graphics [email protected] Published in July 2018 Price: TK 200 US$ 10 ISSN-2413-5445 BOARD of EDITORS Md. Nur-KKhan Adviser Md. Nazmul Hasan Editor Executive Editors Md. Omar Farok Md. Imamul Hossain Research & Publication Advocacy & Networking Aziz Aktar Md. Saiful Islam Monitoring & Documentation Fact findings and Investigation Acknowledgments States are the most responsible authorities to protect the rights of citizens with the help of law enforcement agencies so that people can enjoy their rights. The government is the legitimate custodian and savior of the civil rights of all its citizens. According to social scientists, when a state fails to ensure the protection of human rights of its citizens, it is considered as failed state. The United Nations possesses the authority to monitor the actions of member States for the protection and promotion of human rights around the globe. Bangladesh, as a member of the United Nations and signatory to a large number of international human rights treaties and conventions, has an obligation to ensure the rights of its people. Moreover, a number of universally declared human rights have been guaranteed in Part-III of the Constitution of the People’s Republic of Bangladesh. -

Report on AK Taj Group Masrur M. A. Hoque.Pdf (983.4Kb)
Internship Report on AK TAJ GROUP Prepared for, MD. Tamzidul Islam Assistant Professor BRAC BusinessSchool BRAC University Prepared By, Masrur M. A. Hoque ID # 12164092 Submission Date – 15/12/2015 LETTER OF TRANSMITTAL December 15, 2015 MD. Tamzidul Islam Assistant Professor BRAC BusinessSchool BRAC University Subject: Internship Report. Dear Sir, I would like to thank you for supervising and helping me throughout the semester. With due respect I am submitting a copy of intern report foryourappreciation. I have given my best effort to prepare the report with relevant information that I have collected from an onsite production department which is belongs to a group of company and from other sources during my accomplishthe course. I have the immense pleasure to have the opportunity to study on the marketing practices of AK TAJ Group. There is no doubt that the knowledge I have gathered during the study will help me in real life. For your kind consideration I would like to mention that there might be some errors and mistakes due to limitations of my knowledge. I expect that you will forgive me considering that I am still learner and in the process of learning. Thanking for your time and reviews. Yours faithfully Masrur M. A. Hoque ID-12164092 BRAC Business School BRAC University Acknowledgement The successful completion of this internship might not be possible in time without the help some person whose suggestion and inspiration made it happen. First of all I want to thank my Course Instructor MD. Tamzidul Islam for guiding me during the course. Without his help this report would not have been accomplished. -

List of Upazilas of Bangladesh
List Of Upazilas of Bangladesh : Division District Upazila Rajshahi Division Joypurhat District Akkelpur Upazila Rajshahi Division Joypurhat District Joypurhat Sadar Upazila Rajshahi Division Joypurhat District Kalai Upazila Rajshahi Division Joypurhat District Khetlal Upazila Rajshahi Division Joypurhat District Panchbibi Upazila Rajshahi Division Bogra District Adamdighi Upazila Rajshahi Division Bogra District Bogra Sadar Upazila Rajshahi Division Bogra District Dhunat Upazila Rajshahi Division Bogra District Dhupchanchia Upazila Rajshahi Division Bogra District Gabtali Upazila Rajshahi Division Bogra District Kahaloo Upazila Rajshahi Division Bogra District Nandigram Upazila Rajshahi Division Bogra District Sariakandi Upazila Rajshahi Division Bogra District Shajahanpur Upazila Rajshahi Division Bogra District Sherpur Upazila Rajshahi Division Bogra District Shibganj Upazila Rajshahi Division Bogra District Sonatola Upazila Rajshahi Division Naogaon District Atrai Upazila Rajshahi Division Naogaon District Badalgachhi Upazila Rajshahi Division Naogaon District Manda Upazila Rajshahi Division Naogaon District Dhamoirhat Upazila Rajshahi Division Naogaon District Mohadevpur Upazila Rajshahi Division Naogaon District Naogaon Sadar Upazila Rajshahi Division Naogaon District Niamatpur Upazila Rajshahi Division Naogaon District Patnitala Upazila Rajshahi Division Naogaon District Porsha Upazila Rajshahi Division Naogaon District Raninagar Upazila Rajshahi Division Naogaon District Sapahar Upazila Rajshahi Division Natore District Bagatipara -

A Study on Residential Shift of the Remittance Receiving Households
MURP THESIS A STUDY ON RESIDENTIAL SHIFT OF THE REMITTANCE RECEIVING HOUSEHOLDS KAUSIK DAS KAUSIK DAS DEPARTMENT OF URBAN AND REGIONAL PLANNING BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY DHAKA, BANGLADESH MAY 2010 MAY 2010 A STUDY ON RESIDENTIAL SHIFT OF THE REMITTANCE RECEIVING HOUSEHOLDS By KAUSIK DAS MASTER OF URBAN AND REGIONAL PLANNING DEPARTMENT OF URBAN AND REGIONAL PLANNING BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY DHAKA, BANGLADESH MAY 2010 The thesis titled, “A STUDY ON RESIDENTIAL SHIFT OF THE REMITTANCE RECEIVING HOUSEHOLDS” submitted by Kausik Das, Roll No: 100615032(F),Session: October 2006, has been accepted as satisfactory in partial fulfillment of the requirements for the degree of MASTER OF URBAN AND REGIONAL PLANNING (MURP) on 4 May, 2010 BOARD OF EXAMINERS Md. Musleh Uddin Hasan Assistant Professor Department of Urban and Regional Planning, BUET Chairman &Supervisor Dr. Sarwar Jahan Professor and Head Department of Urban and Regional Planning, BUET Member(Ex-Officio) Dr. Shakil Akther Associate Professor Department of Urban and Regional Planning, BUET Member Dr. Chowdhury Rafiqul Abrar Professor Department of International Relations, Member (External) Dhaka University Director RMMRU, Dhaka CANDIDATE’S DECLARATION It is hereby declared that this thesis or any part of it has not been submitted elsewhere for the award of any degree or diploma. Kausik Das Student No. 100615032 URP, BUET, Dhaka Dedicated to My parents TABLE OF CONTENT Page No. List of Tables iv List of Figures v List of Maps v Abbreviation -
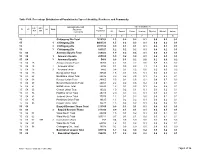
Chittagong C09.Pdf
Table C-09: Percentage Distribution of Population by Type of disability, Residence and Community Administrative Unit Type of disability (%) UN / MZ / Total ZL UZ Vill RMO Residence WA MH Population Community All Speech Vision Hearing Physical Mental Autism 1 2 3 4 5 6 7 8 9 10 15 Chittagong Zila Total 7616352 1.3 0.2 0.2 0.1 0.5 0.2 0.1 15 1 Chittagong Zila 4463723 1.5 0.2 0.3 0.1 0.6 0.2 0.1 15 2 Chittagong Zila 2971102 0.9 0.1 0.1 0.1 0.4 0.1 0.1 15 3 Chittagong Zila 181527 1.2 0.2 0.2 0.1 0.4 0.2 0.1 15 04 Anowara Upazila Total 259022 1.9 0.2 0.4 0.1 0.8 0.3 0.1 15 04 1 Anowara Upazila 253556 1.9 0.2 0.4 0.1 0.8 0.3 0.1 15 04 3 Anowara Upazila 5466 0.8 0.1 0.2 0.0 0.2 0.0 0.2 15 04 15 Anowara Union Total 10260 4.2 0.3 2.2 0.6 0.8 0.2 0.2 15 04 15 1 Anowara Union 4794 8.1 0.5 4.5 1.1 1.5 0.3 0.3 15 04 15 3 Anowara Union 5466 0.8 0.1 0.2 0.0 0.2 0.0 0.2 15 04 19 Bairag Union Total 30545 1.8 0.1 0.5 0.1 0.6 0.3 0.1 15 04 28 Barakhain Union Total 28836 3.0 0.2 0.5 0.1 1.8 0.3 0.1 15 04 38 Barasat Union Total 28865 2.5 0.2 0.6 0.2 0.8 0.7 0.2 15 04 47 Burumchhara Union Total 20061 2.2 0.2 0.6 0.2 1.0 0.1 0.1 15 04 57 Battali Union Total 23630 1.3 0.1 0.2 0.1 0.5 0.2 0.1 15 04 66 Chatari Union Total 19022 1.0 0.2 0.1 0.1 0.3 0.2 0.1 15 04 76 Haildhar Union Total 25315 2.0 0.4 0.3 0.1 0.7 0.3 0.1 15 04 81 Juidandi Union Total 17575 1.2 0.1 0.2 0.0 0.6 0.1 0.1 15 04 85 Paraikora Union Total 19635 1.3 0.2 0.2 0.1 0.6 0.2 0.1 15 04 95 Roypur Union Total 35278 1.5 0.2 0.2 0.1 0.6 0.2 0.1 15 06 Bayejid Bostami Thana Total 211355 0.9 0.1 0.1 0.1 0.4 0.1 0.1 15 06 2 Bayejid Bostami Thana 211355 0.9 0.1 0.1 0.1 0.4 0.1 0.1 15 06 01 Ward No-01 Total 29209 1.5 0.1 0.2 0.1 0.9 0.1 0.1 15 06 02 Ward No-02 Total 103314 0.7 0.1 0.1 0.0 0.3 0.1 0.1 15 06 02 998 2 Cantonment (Part) 758 0.4 0.0 0.0 0.0 0.1 0.0 0.3 15 06 03 Ward No-03 Total 68794 1.2 0.1 0.2 0.1 0.5 0.2 0.1 15 06 98 Chittagang Cnt.