BIO 311 Plant Structure and Development Lab Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Managing Lenticel Breakdown – Don’T Get Caught by the Snowball
Managing Lenticel Breakdown – Don’t get caught by the snowball Rob Blakey Stemilt Growers LLC, Wenatchee, WA (Formerly Washington State University, Prosser, WA) [email protected] Lenticel breakdown and other lenticel‐related disorders can be serious quality defects on apples. As with any fruit defect, it is important to correctly diagnose the defect to adapt management practices accordingly. Lenticel breakdown can be misdiagnosed as calcium burn, mild lenticel blotch pit, blister spot (Pseudomonas syringae), lenticel sunburn, bitter pit, and early stage speck rot (Phacidiopycnis washingtonensis) in Washington, etc. For diagnosis assistance, the reader is referred to the Lenticel Related Disorders Matrix from WSU (bit.ly/LenticelDisorders). Lenticel breakdown symptoms express after postharvest handling, but damage is set‐up pre‐ harvest when fruit grow rapidly and micro‐cracks develop in the fruit cuticle the epidermis and hypodermis (skin) cells are exposed to aggravation and desiccation. Symptoms are associated with lenticels because these cuticle micro‐cracks are often associated with lenticels and the lenticel provides an access point for aggravants and water egress which can eventually result in cell death and pitting around the lenticel. Aggravants may be dust, environmental protectant spray particles, salts dissolved in water, or agro‐chemicals. Lower risk fruit are generally: smaller, firmer, have less starch clearing, lower soluble solids, higher titratable acidity, lower K, Mg, and N, and higher Ca. Lenticel breakdown is most typically seen in Gala and Fuji in Washington. Lenticel breakdown is caused by a number of factors, with these factors accumulating over time (i.e. ‘snowballing’ and finally resulting in severe losses from lenticel breakdown. -

Lenticels of Different Plant Species
University of Pretoria etd – Bezuidenhout, J L J (2005) CHAPTER 3 LENTICEL ONTOGENY OF ‘TOMMY ATKINS’, ‘KEITT’ AND ‘KENT’ FRUIT ABSTRACT Lenticels differentiate from existing stomata that lose their function and protrude above the fruit surface as a result of rapid anticlinal cell divisions in the epidermis of the exocarp. Based on the comparative study between different mango cultivars and mature marula fruit, it seems as if the absence of a cork cambium and cork cells in the mango lenticel could be one of the most important reasons for lenticel discolouration. An interaction between naturally occurring pigments and sap from the resin ducts in the exocarp appears to be another contributing factor for lenticel discolouration. 3.1 INTRODUCTION Lenticels can be found on the surface of stems, old roots and on several fruit types, including apples, pears, avocados and mangos (Dietz et al., 1988). In the absence of stomata will the lenticels take over the vitally important process of gaseous exchange needed for photosynthesis, respiration and transpiration (Mauseth, 1988). Postharvest discolouration of mango lenticels is a serious problem, since the resultant black markings on the fruit skin are unacceptable to consumers, consequently depreciating the economic value of the fruit (O’Hare and Prasad, 1992). The degree of lenticel discolouration may vary in different mango cultivars. In South Africa, ‘TA’ and ‘Keitt’ are two of the most important cultivars susceptible to lenticel discolouration, whereas ‘Kent’ is not known to problematic in that aspect. According to Dietz et al. (1988), mango fruit lenticels may develop from either pre-existing stomata, or from rupturing of the epidermis. -

Preharvest Lipophilic Coatings Reduce Lenticel Breakdown Disorder in 'Gala' Apples
(peel) arc often linked to climatic conditions during the growing sea- son and are initiated when a partic- ular metabolic system(s) exhibits strcss- induced hysteresis. These include russet, staining, cracking, splitting, flecking, bitter pit, blotch, lenticel marking, radiation injury, delayed sunscald, superficial scald, and Soft scald (Mehcriuk et al., 1994; Pierson et al., 1971; Porritt et al., 1982). Together, these disor- ders may render unmarketable as niuch as 20% of total production. Considering that the value of apples in Washington state alone in 2006 was $1.4 billion (National Agricul- Preharvest Lipophilic Coatings Reduce tural Statistical Service, 2007), reduc- Lenticel Breakdown Disorder in 'Gala' Apples ing the loss due to physiological disorders is of significant economic importance. " 3 Eric A. Curry '14 , Carolina Torrcs , and Luis Ncubaucr Since 2000, lenticel breakdown disorder (LR) has been a high priority area for research investigations in the ADDITIONAL INDEX wons. Mt1us xdomestici, physiological disorder, storage, cuticle, microcracking, wax, lipids, 'Fuji', 'Granny Smith', 'Golden Delicious' and apple growing regions of the United States. LB symptoms are not SUMMARY. Lenticel breakdown disorder (LB), most prevalent on 'Gala' (Malus x visible at harvest nor are they usually domestiot) apples, especially in arid regions, has also been observed on other apparent on unprocessed fruit after common cultivars. Depending on the preharvest environment, fruit maturity, and storage. It is usually after typical frLlit length of storage, LB usually appears as one or more round, darkened pits, centered ymp- on a lenticel, ranging in diameter from 1 to 8 mm. Symptoms are not visible at processing and packing that s harvest nor are they usually apparent on unprocessed fruit after storage. -

Latin Derivatives Dictionary
Dedication: 3/15/05 I dedicate this collection to my friends Orville and Evelyn Brynelson and my parents George and Marion Greenwald. I especially thank James Steckel, Barbara Zbikowski, Gustavo Betancourt, and Joshua Ellis, colleagues and computer experts extraordinaire, for their invaluable assistance. Kathy Hart, MUHS librarian, was most helpful in suggesting sources. I further thank Gaylan DuBose, Ed Long, Hugh Himwich, Susan Schearer, Gardy Warren, and Kaye Warren for their encouragement and advice. My former students and now Classics professors Daniel Curley and Anthony Hollingsworth also deserve mention for their advice, assistance, and friendship. My student Michael Kocorowski encouraged and provoked me into beginning this dictionary. Certamen players Michael Fleisch, James Ruel, Jeff Tudor, and Ryan Thom were inspirations. Sue Smith provided advice. James Radtke, James Beaudoin, Richard Hallberg, Sylvester Kreilein, and James Wilkinson assisted with words from modern foreign languages. Without the advice of these and many others this dictionary could not have been compiled. Lastly I thank all my colleagues and students at Marquette University High School who have made my teaching career a joy. Basic sources: American College Dictionary (ACD) American Heritage Dictionary of the English Language (AHD) Oxford Dictionary of English Etymology (ODEE) Oxford English Dictionary (OCD) Webster’s International Dictionary (eds. 2, 3) (W2, W3) Liddell and Scott (LS) Lewis and Short (LS) Oxford Latin Dictionary (OLD) Schaffer: Greek Derivative Dictionary, Latin Derivative Dictionary In addition many other sources were consulted; numerous etymology texts and readers were helpful. Zeno’s Word Frequency guide assisted in determining the relative importance of words. However, all judgments (and errors) are finally mine. -

An Access-Dictionary of Internationalist High Tech Latinate English
An Access-Dictionary of Internationalist High Tech Latinate English Excerpted from Word Power, Public Speaking Confidence, and Dictionary-Based Learning, Copyright © 2007 by Robert Oliphant, columnist, Education News Author of The Latin-Old English Glossary in British Museum MS 3376 (Mouton, 1966) and A Piano for Mrs. Cimino (Prentice Hall, 1980) INTRODUCTION Strictly speaking, this is simply a list of technical terms: 30,680 of them presented in an alphabetical sequence of 52 professional subject fields ranging from Aeronautics to Zoology. Practically considered, though, every item on the list can be quickly accessed in the Random House Webster’s Unabridged Dictionary (RHU), updated second edition of 2007, or in its CD – ROM WordGenius® version. So what’s here is actually an in-depth learning tool for mastering the basic vocabularies of what today can fairly be called American-Pronunciation Internationalist High Tech Latinate English. Dictionary authority. This list, by virtue of its dictionary link, has far more authority than a conventional professional-subject glossary, even the one offered online by the University of Maryland Medical Center. American dictionaries, after all, have always assigned their technical terms to professional experts in specific fields, identified those experts in print, and in effect held them responsible for the accuracy and comprehensiveness of each entry. Even more important, the entries themselves offer learners a complete sketch of each target word (headword). Memorization. For professionals, memorization is a basic career requirement. Any physician will tell you how much of it is called for in medical school and how hard it is, thanks to thousands of strange, exotic shapes like <myocardium> that have to be taken apart in the mind and reassembled like pieces of an unpronounceable jigsaw puzzle. -

Running Head 'Biology of Mangroves'
BIOLOGY OF MANGROVES AND MANGROVE ECOSYSTEMS 1 Biology of Mangroves and Mangrove Ecosystems ADVANCES IN MARINE BIOLOGY VOL 40: 81-251 (2001) K. Kathiresan1 and B.L. Bingham2 1Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai 608 502, India 2Huxley College of Environmental Studies, Western Washington University, Bellingham, WA 98225, USA e-mail [email protected] (correponding author) 1. Introduction.............................................................................................. 4 1.1. Preface........................................................................................ 4 1.2. Definition ................................................................................... 5 1.3. Global distribution ..................................................................... 5 2. History and Evolution ............................................................................. 10 2.1. Historical background ................................................................ 10 2.2. Evolution.................................................................................... 11 3. Biology of mangroves 3.1. Taxonomy and genetics.............................................................. 12 3.2. Anatomy..................................................................................... 15 3.3. Physiology ................................................................................. 18 3.4. Biochemistry ............................................................................. 20 3.5. Pollination -

Ultrastructure of the Pneumatophores of the Mangrove a Vicennia Marina
358 S.Afr.J.Bot., 1992, 58(5): 358 - 362 Ultrastructure of the pneumatophores of the mangrove A vicennia marina Naomi Ish-Shalom-Gordon* and Z. Dubinsky Department of Life Sciences, Bar-lian University, Ramat-Gan 52900, Israel ·Present address: Golan Research Institute, P.O. Box 97, Qazrin 12900, Israel Received 24 February 1992; revised 3 June 1992 Pneumatophores of Avicennia marina (Forssk.) Vierh. were studied by scanning electron microscopy (SEM) in order to relate their ultrastructure to their function as air conduits. The path of air from the atmosphere through the lenticel into the aerenchyma and then to the horizontal root is described. Different states of the lenticels were observed (closed, partially opened, fully opened), and are suggested as developmental stages of the lenticel. The nature of the complementary cells and their role in the aeration function of the lenticel are characterized. Die pneumatofore van Avicennia marina (Forsh.) Vierh . is deur skandeerelektronmikroskopie bestudeer om die verband tussen die ultrastruktuur en hu"e funksie as lug wee vas te stel. Die pad van lug vanaf die atmosfeer deur die lentisel na die aerenchiemweefsel en daarna na die horisontale wortel word beskryf. Verski"ende toestande van die lentise"e (geslote, gedeeltelik oop en volledig oop), wat waarskynlik ontwikkelingstadia van die lentisel is, is waargeneem . Die aard van die komplementere selle en hulle rol in die belugtingsfunksie van die lentisel word gekarakteriseer. Keywords: Avicennia marina, lenticel, mangrove, pneumatophore, ultrastructure. Introduction short and long distances from the main trunk of the plant Avicennia marina (Forssk.) Vierh. (Verbenaceae) is a (20 cm and 12 m, respectively) were used. -

Preventing Fruit Rots and Spray-Associated Lenticel Issues in Apples
Preventing Fruit Rots and Spray-associated Lenticel Issues in Apples Srdjan Acimovic, PhD David Rosenberger, PhD Hudson Valley Research Laboratory Empire EXPO, Syracuse 17 Jan 2018 Outline • Preventing fruit rots • Black rot • White rot • Bitter rot • Management • Spray lenticel issues • Lenticel look & function • Known spray causes • Unknown • Distinguish • What can you do? Preventing fruit rots - Fungi - • Black rot (Botryosphaeria obtusa) • White rot (Botryosphaeria dothidea) • Bitter rot (Colletotrichum spp.) • Gray mold (Botrytis cinerea) –will not cover today • All these fungi can later become storage- decays Photo by D. Rosenberger Black Rot - B. obtusa - Red Delicious Cortland - Frogeye leaf spot Gala Photo by D. Rosenberger Black Rot - B. obtusa - • Overwinters in cankers, mummy fruitlets, dead bark, brush, trunk cankers (internal fungi decay), multiple hosts • Infects fruit at warm rains (Spring: perithecia, Summer: pycnidia) • Infects from petal fall - Harvest Photo by D. Rosenberger • Forms dormant infections • Fruit ripening activates the fungus • It is a firm rot, mostly drier • It inhabits fruitlet mummies after thinning Photo by D. Rosenberger • Lenticel infections occur on semi-mature fruit during summer • These infections are visible as lenticel spots • Later it can activate and continue to cause decay in storage • Lenticel spots caused by white rot, black rot, and bitter rot possible if spray residue was lost before harvest. White rot - B. dothidea - Photo by D. Rosenberger White rot - B. dothidea - • Overwintering same -

2009 KERN COUNTY POTATO VARIETY TRIAL Joe Nunez and Jed Dubose
University of California Cooperative Extension KERN VEGETABLE CROPS Kern County • 1031 S. Mt. Vernon Avenue • Bakersfield, CA 93307 • 661-868-6222 Summer 2009 2009 KERN COUNTY POTATO VARIETY TRIAL Joe Nunez and Jed Dubose We completed the 2009 Kern County Potato Variety Trial in June with a successful field day with many excellent varieties to look over. Below are the results of the variety trial with some very good yielding varieties. A95109 is now named Classic Russet which has done well in our trial for several years. Pacific Russet is a newly released variety also that some growers here had interest in. Both Classic Russet and Pacific Russet look like potential replacements for Russet Norkotah. Overall, there are many varieties to consider trying on a small acreage basis. I have pictures of all these varieties including from years past trials. Please contact me if you would like a copy. We would like to thank Kevin and Dennis Johnston of Johnston Farms for their generosity in hosting the trial the past several years. Kern County Replicated Trial cwt Entry Total No. Variety 0-4 4-6 6-10 11> Culls Weight Harvestable 31 MSQ176-5 19.2 51.3 117.4 28.2 2.0 218.1 216.0 32 MSQ070-1 76.0 177.0 166.2 12.3 0.0 431.4 431.4 33 MSL292-A 34.1 104.5 126.8 14.3 0.8 280.6 279.7 34 MSQ279-1 27.8 80.4 176.0 80.7 12.3 377.1 364.9 35 MSL268-D 77.0 162.5 233.0 18.8 1.4 492.7 491.3 36 MSM182-1 73.9 196.6 198.3 11.4 2.9 483.1 480.2 37 ARGOS 66.0 100.3 286.3 71.3 3.5 527.2 523.7 38 A95109-1 29.8 71.7 186.4 50.0 20.2 358.1 337.9 39 AO96160-3 102.5 81.5 164.8 -

Tree Anatomy I
ADVANCED TREE BIOLOGY: TREE ANATOMY I by Dr. Kim D. Coder, Professor of Tree Biology & Health Care W arnell School of Forestry & Natural Resources, University of Georgia Abstract: Professional tree health care providers and tree managers should always use the proper terms and definitions for tree components, parts, and growth patterns. Understanding proper scientific names for anatomical components is critical in identifying and describing tree parts and problems. This workshop is an advanced technical look at tree anatomy and morphology at the macroscopic levels (<15X) in above ground structures. Concentration will be on identifying and naming common tree growth forms, and visible tree tissues and their organization. Coverage includes twig, branch, stem, and periderm anatomy, along with identifying features visible with the naked eye or under low magnification. Can you tell one part from another? A certificate of completion will be provided. Workshop Outline: 1. INTRODUCTION – DEFINING TREES 2. GENERAL CROWN FORM 3. MERISTEMS 4. BUDS AND GROWING POINTS 4A. BUD DEFINITIONS 4B. BUD CONTENTS 4C. GROWING POINT FORMS 5. TWIGS 5A. TWIG FORM 5B. TWIG CICATRICES 6. TWIG / BRANCH / STEM 7. STEM 7A. STEM CROSS-SECTION 7B. STEM FORM 7C. SHOOT GROWTH PATTERNS Page 1 of 32 7D. SECONDARY XYLEM & PHLOEM 9D1. GYMNOSPERMS 9D2. ANGIOSPERMS 7E. XYLEM INCREMENT TYPES 7F. SAPWOOD / HEARTWOOD 7G. BRANCH ATTACHMENT 7H. PRUNING ANATOMY 8. PERIDERM 8A. PERIDERM DEFINITIONS 8B. PERIDERM FORM 9. SELECTED LITERATURE WORKSHOP MANUAL GUIDE INTRODUCTION morphology = study of external shape, form, and structure seed bearing plants = angiosperms & gymnosperms (both part of Spermatophytes) flowering plants = angiosperms angiosperms = flowering plants which have seeds enclosed in carpels (fruit) eudicots = 75% of angiosperms – modern dicots = 3% of angiosperms – ancient (Magnoliosida) monocots = 22% of angiosperms gymnosperms = seed plants with ovules not in an ovary but exposed to the environment (i.e. -
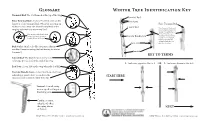
Winter Tree Identification
Glossary Winter Tree Identification Key Terminal Bud: The bud formed at the tip of the twig Terminal Bud False Terminal Bud: A lateral bud that assumes the Bud Scales function of the terminal bud. When the growing tip False Terminal Bud withers or falls away, the closest lateral bud to the Lateral Bud Note the part of the branch twig tip substitutes as a terminal bud from last year’s growth that extends beyond the base of Note the part of the branch from the bud. This helps you last year’s growth that extends Vascular Bundle Scar determine whether a bud beyond the base of the bud. is a false terminal bud. It may be very noticeable or may be difficult to see Leaf Scar without a hand lens. Bud Scales: Small scale-like structures that are modified leaves covering the bud during its winter dormancy Lenticel KEY TO TERMS Lateral Bud: The buds formed on the side of a twig, not the bud at the end of the twig 1. Leaf scars opposite...Got to 2 OR 1. Leaf scars alternate...Go to 3 Leaf Scar: A scar left on the twig when the leaf falls Vascular Bundle Scars: A small mark on a leaf scar indicating a point where a vein from the START HERE leaf was once connected with the stem Lenticel: A small corky area or speck serving as a breathing pore Catkin: A dense, cylindrical, often drooping cluster NEXT of flowers LEAF Winter Tree ID Key ©2014 www.leafprogram.org LEAF Winter Tree ID Key ©2014 www.leafprogram.org Vascular bundle scars are very numerous and joined in a line; buds Fraxinus Ash Betula Birch 2 15 3 bud scales are broadly rounded; twigs are fairly stout see 2a-c below See 15 a-c below 2a. -

A Study of Micro-Morphology of Grape Berry Surface During Their Development with Special Reference to Stoma
J. Japan. Soc. Hort. Sci. 49(1) :1-7. 1980. A Study of Micro-morphology of Grape Berry Surface during Their Development with Special Reference to Stoma Sholchl NAKAGAWA,Harukl KOMATSUand Ei ji YUDA Department of Horticulture, University of Osaka Prefecture, Sakai, Osaka 591 Summary Using seven grape cultivars, `Concord', `Campbell Early', `Delaware', `Takao', `Kyoho' , `Muscat of Alexandria' and `Koshu', changes in the stomatal morphology of berry epidermis accompanied by the growth were observed under scanning electron microscope (SEM). Average number of stomata on the berry surface of `Muscat of Alexandria' at anthesis was 16, that of `Campbell Early', `Delaware', `Kyoho' and `Koshu' was around 12, and those of `Concord' and `Takao' were 7.0 and 2. 4, respectively. The size of stomata in grape berry epidermis at anthesis was not differed among the cultivars, and was 2025 p in width and around 25 p in length. Twenty days before anthesis the guard cells were not yet differentiated, but distinguishable just prior to anthesis. Furrowed feature was seen on the inner wall of opening stomata in this period. After fertilization cracks occurred in the epidermis circulating around some stomata with berry growth, and cork layers developed beneath such stomata, which sometimes protruded from the epidermal layer forming lenticel-like structure. From these observations what were visibly seen as brown specks on grape berry surface at near maturity were confirmed as stomata and the suberized cells adjacent to them. in the grape berry epidermis. On the con- Introduction trary, Kozma P. (4) reported a small number Fruit epidermis is a protecting tissue, of stomata was present in young berries and consisting usually of one layer of cells ac- they would close down in 2-3 weeks after companied sometimes by trichomes or sto- bloom and developed into lenticels.