Droplet Microfluidics for Chip-Based Diagnostics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Digital Microfluidics
ON-CHIP BLADE FOR ACCURATE SPLITTING OF DROPLETS IN LIGHT-ACTUATED DIGITAL MICROFLUIDICS Shao Ning Pei1, and Ming C. Wu1 1Berkeley Sensor & Actuator Center (BSAC), Electrical Engineering and Computer Sciences, University of California, Berkeley, USA ABSTRACT We report on a new technique to slice droplets with integrated “Teflon blades” in the light-actuated digital microfluidic platforms. The droplet splitting ratio (10% to 90%) can be accurately controlled by the blade position. Uniform droplet generation (75.2nL with 2% variation) has been demonstrated by repeatedly slicing an elongated mother droplet. Additionally, by applying this technique to a concentrated sample of food dye in droplet, a 5-step serial dilution with consistent sample dilution factor of 6x, and total dilution factor of 65 = 7776, has been achieved. KEYWORDS: electrowetting, optoelectrowetting, digital microfluidics, droplet microfluidics, EWOD INTRODUCTION Digital microfluidics has been an exciting development in the past decade with many promising applications ranging from drug discovery to electronic cooling. Traditional digital microfluidics uses array of electrodes to transport droplets [1, 2]. Recently, we reported on a light-actuated digital microfluidics device [3, 4], which replaces the physically patterned electrodes in the traditional device with a continuous film of photoconductor. “Virtual” electrodes are created on the device surface when optical patterns are shone on the device. With this methodology, real-time, parallel droplet control can occur without the need for complex electrode addressing schemes. Splitting droplet is an integral operation for digital microfluidics as it is required for both drawing droplets from reservoirs and controlling droplet volume in multi-step assays, titration, and multiplexing bioassays. -

Biomems Literature by Year Prof
BioMEMS Literature by Year Prof. Steven S. Saliterman 1. Xu M, Obodo D, Yadavalli VK. The design, fabrication, and applications of flexible bio- sensing devices. Biosensors & Bioelectronics. 2019;124:96-114. 2. Wongkaew N, Simsek M, Griesche C, Baeumner AJ. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chemical Reviews. 2019;119(1):120-194. 3. Wang MH, Yin HS, Zhou YL, et al. Photoelectrochemical biosensor for microRNA detec- tion based on a MoS2/g-C3N4/black TiO2 heterojunction with Histostar@AuNPs for signal amplification. Biosensors & Bioelectronics. 2019;128:137-143. 4. Wang JS, Hui N. Electrochemical functionalization of polypyrrole nanowires for the de- velopment of ultrasensitive biosensors for detecting microRNA. Sensors and Actuators B-Chemical. 2019;281:478-485. 5. Sun EWL, Martin AM, Young RL, Keating DJ. The Regulation of Peripheral Metabolism by Gut-Derived Hormones. Frontiers in Endocrinology. 2019;9. 6. Soler M, Huertas CS, Lechuga LM. Label-free plasmonic biosensors for point-of-care di- agnostics: a review. Expert Review of Molecular Diagnostics. 2019;19(1):71-81. 7. Soler M, Huertas CS, Lechuga LM. Label-free plasmonic biosensors for point-of-care di- agnostics: a review. Expert Review of Molecular Diagnostics. 2019;19(1):71-81. 8. Sola L, Damin F, Chiari M. Array of multifunctional polymers for localized immobilization of biomolecules on microarray substrates. Analytica Chimica Acta. 2019;1047:188-196. 9. Seidi S, Ranjbar MH, Baharfar M, Shanehsaz M, Tajik M. A promising design of microflu- idic electromembrane extraction coupled with sensitive colorimetric detection for col- orless compounds based on quantum dots fluorescence. -

Digital Microfluidics As a Reconfiguration Mechanism for Antennas
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 8-2013 Digital Microfluidics As A Reconfiguration Mechanism For Antennas Yasin Damgaci Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Electrical and Electronics Commons Recommended Citation Damgaci, Yasin, "Digital Microfluidics As A Reconfiguration Mechanism For Antennas" (2013). All Graduate Theses and Dissertations. 1761. https://digitalcommons.usu.edu/etd/1761 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. DIGITAL MICROFLUIDICS AS A RECONFIGURATION MECHANISM FOR ANTENNAS by Yasin Damgaci A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Electrical Engineering Approved: Dr. Bedri A. Cetiner Dr. Jacob Gunther Major Professor Committee Member Dr. Reyhan Baktur Dr. Edmund Spencer Committee Member Committee Member Dr. T.C. Shen Dr. Mark R. McLellan Committee Member Vice President for Research and Dean of the School of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2013 ii Copyright c Yasin Damgaci 2013 All Rights Reserved iii Abstract Digital Microfluidics as a Reconfiguration Mechanism for Antennas by Yasin Damgaci, Doctor of Philosophy Utah State University, 2013 Major Professor: Dr. Bedri A. Cetiner Department: Electrical and Computer Engineering This dissertation work concentrates on novel reconfiguration technologies, including design, microfabrication, and characterization aspects with an emphasis on their applica- tions to multifunctional reconfigurable antennas. -

Publication List Ming C
Publications Ming C. Wu Publication List Ming C. Wu (PDF files of most publications are available at http://www.photonics.ucla.edu/publications.php ) Book Chapters B1. M.C. Wu, J.-C. Tsai, W. Piyawattanametha, and P.R. Patterson, “Optical MEMS and Nano- Photonics,” in MEMS/NEMS Handbook (translated into Chinese), Zhou Zhaoying, Editor, Chinese Science Publishing Company, to be published in 2005. B2. M. C. Wu and P. R. Patterson, “Free-Space Optical MEMS,” in MEMS Handbook, korvink and O. Paul, Editors, William Andrew Publishing, NY, to be published in 2004. B3. P. K. L. Yu and M. C. Wu, “Photodiodes for High Performance Analog Links,” in RF Photonic Technology in Optical Fiber Links, W. S. C. Chang, Editor, Cambridge University Press, Cambridge, UK, 2002. B4. M. C. Wu, S. S. Lee, L. Fan, and G. D. Su, “Optical MEMS: Huge Possibilities for Lilliputian- sized Devices,” in Trends in Optics and Photonics (TOPS), Vol. 23, H. Haus, Editor, Optical Society of America, Washington DC, October 1998. B5. M. C. Wu and Y. K. Chen, “Monolithic Colliding Pulse Mode-Locked Diode Lasers,” in Compact Sources for Ultrashort Pulses, I. Duling, III, Editor, pp. 381-424, Cambridge University Press, Cambridge, UK, 1995. Archival Journal Publications J1. J-A. Paik, S-K. Fan, H. Chang, C-J. Kim, M.C. Wu and B. Dunn, “Development of Spin Coated Mesoporous Oxide Films for MEMS Structures,” accepted for publication in J. Electroceramics J2. Dooyoung Hah, Patterson PR, Nguyen HD, Toshiyoshi H, Wu MC. “Theory and experiments of angular vertical comb-drive actuators for scanning micromirrors,” IEEE Journal of Selected Topics in Quantum Electronics, vol.10, no.3, May-June 2004, pp.505-13. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Microfluidic and Holographic Microscopy Integrated Optoelectronic Tweezers For Single-cell Applications Permalink https://escholarship.org/uc/item/6m39t35w Author Huang, Kuo-Wei Publication Date 2012 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Microfluidic and Holographic Microscopy Integrated Optoelectronic Tweezers For Single-cell Applications A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Mechanical Engineering by Kuo-Wei Huang 2013 © Copyright by Kuo-Wei Huang 2013 ABSTRACT OF THE DISSERTATION Microfluidic and Holographic Microscopy Integrated Optoelectronic Tweezers For Single-cell Applications By Kuo-Wei Huang Doctor of Philosophy in Mechanical Engineering University of California, Los Angeles, 2013 Professor Pei-Yu Chiou, Chair Optoelectronic tweezers (OET), combining the advantages of optical and dielectrophoretic (DEP) manipulation, has been widely used in laboratories around the world. Despite being a useful platform for single-cell manipulation, OET still suffers from two limitations, the difficulty of being integrated with microfluidic components and the lack of large-area real-time detection function. The two issues restrain OET from complicated single-cell analysis and large-area automated manipulation. To integrate the PDMS-based microfluidic components with OET, two techniques are purposed in this research, SWNT-embedded electrodes and 3D PDMS membranes. The first technique uses SWNT thin films as transparent and conductive electrodes for OET inside multilayer PDMS channels. 3D microfluidic technique makes PDMS membranes to be a multilayer network between two rigid OET substrates. Based on these two new fabrication methods, novel microfluidic OET platforms have been developed to enable single or ii multiple cell manipulation and to accomplish multi-steps tasks. -

Designing Splicing Digital Microfluidics Chips Based
micromachines Article Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane Haoqiang Feng 1,2, Zichuan Yi 1,*, Ruizhi Yang 2, Xiaofeng Qin 2, Shitao Shen 2, Wenjun Zeng 1,2, Lingling Shui 1,2, Guofu Zhou 2 and Chongfu Zhang 1 1 College of Electron and Information, University of Electronic Science and Technology of China, Zhongshan Institute, Zhongshan 528402, China; [email protected] (H.F.); [email protected] (W.Z.); [email protected] (L.S.); [email protected] (C.Z.) 2 South China Academy of Advanced Optoelectronics, South China Normal University, Guangzhou 510006, China; [email protected] (R.Y.); [email protected] (X.Q.); [email protected] (S.S.); [email protected] (G.Z.) * Correspondence: [email protected]; Tel.: +86-0760-8831-4612 Received: 11 November 2020; Accepted: 29 November 2020; Published: 30 November 2020 Abstract: As a laboratory-on-a-chip application tool, digital microfluidics (DMF) technology is widely used in DNA-based applications, clinical diagnosis, chemical synthesis, and other fields. Additional components (such as heaters, centrifuges, mixers, etc.) are required in practical applications on DMF devices. In this paper, a DMF chip interconnection method based on electrowetting-on-dielectric (EWOD) was proposed. An open modified slippery liquid-infused porous surface (SLIPS) membrane was used as the dielectric-hydrophobic layer material, which consisted of polytetrafluoroethylene (PTFE) membrane and silicone oil. Indium tin oxide (ITO) glass was used to manufacture the DMF chip. In order to test the relationship between the splicing gap and droplet moving, the effect of the different electrodes on/off time on the minimum driving voltage when the droplet crossed a splicing gap was investigated. -

Continuous Electrowetting in Passivating and Non-Passivating Systems" (2013)
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School January 2013 Continuous Electrowetting in Passivating and Non- passivating Systems Mehdi Khodayari University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the Mechanical Engineering Commons Scholar Commons Citation Khodayari, Mehdi, "Continuous Electrowetting in Passivating and Non-passivating Systems" (2013). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/4708 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Continuous Electrowetting in Passivating and Non-passivating Systems by Mehdi Khodayari A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Mechanical Engineering College of Engineering University of South Florida Co-Major Professor: Nathan B. Crane, Ph.D. Co-Major Professor: Alex A. Volinsky, Ph.D. Delcie Durham, Ph.D. Matthias Batzill, Ph.D. Alberto A. Sagüés, Ph.D. Date of Approval: July 3, 2013 Keywords: Reliable Electrowetting, Symmetric Electrowetting, Electrochemical Diodes, Electrostatic Force, Titanium, Aluminum Copyright © 2013, Mehdi Khodayari ACKNOWLEDGMENTS I would like to express my gratitude to Professor Nathan B. Crane and Professor Alex A. Volinsky for all of their scientific and financial support during my Ph.D. study at the University of South Florida. Professor Crane was my immediate advisor and from him I learned how a dedicated advisor would be. Professor Volinsky was my great mentor and advisor too. -

ANTTI-JUHANA MÄKI OPTICALLY INDUCED ELECTRIC FIELDS and THEIR USE in MICROFLUIDICS and CELL MANIPULATION APPLICATIONS Master of Science Thesis
ANTTI-JUHANA MÄKI OPTICALLY INDUCED ELECTRIC FIELDS AND THEIR USE IN MICROFLUIDICS AND CELL MANIPULATION APPLICATIONS Master of Science Thesis Examiners: Prof. Pasi Kallio, PhD Pekka Ronkanen The topic and the examiners have been approved by the Faculty of Automation, Mechanical and Materials Engineering Council meeting on 09.12.2009 II ABSTRACT TAMPERE UNIVERSITY OF TECHNOLOGY Master’s Degree Programme in Automation Technology MÄKI, ANTTI-JUHANA: Optically Induced Electric Fields and Their Use in Microfluidics and Cell Manipulation Applications Master of Science Thesis, 98 pages, 9 appendix pages June 2010 Major: Microsystem Technology Examiners: Professor Pasi Kallio and PhD Pekka Ronkanen Keywords: Droplet manipulation, digital microfluidics, electrowetting, optoelectrowetting, modelling This thesis is a part of OPTIMI (Optically actuated microfluidics) project supported by Academy of Finland. The participants of the project were Tampere University of Technology (TUT) and Helsinki University of Technology (HUT). The goal of the project was to develop a flexible biomedical microfluidic system composed from completely optically induced components. Electrowetting is a mechanism to transport microscale droplets electrically. In electrowetting-based manipulation, droplet is moved by locally modifying the surface tension of liquid. In this thesis, optically induced electrowetting, named as optoelectrowetting, that is using a photoconductive surface for addressing electric fields used for electrowetting, is presented. Optoelectrowetting provides a flexible, illumination-based creation of electric fields that are used to control surface tension of liquid. The scope of this thesis work is to develop a system where the optoelectrowetting mechanism could be implemented to enable manipulation of microliter droplets. This thesis focuses on modelling the optoelectrowetting device in order to provide the requirements for effective optoelectrowetting system. -

(12) United States Patent (10) Patent No.: US 8,753,498 B2 Chuang Et Al
USOO8753498B2 (12) United States Patent (10) Patent No.: US 8,753,498 B2 Chuang et al. (45) Date of Patent: Jun. 17, 2014 (54) OPEN OPTOELECTROWETTING DROPLET (56) References Cited ACTUATION DEVICE AND METHOD U.S. PATENT DOCUMENTS Inventors: Han-Sheng Chuang, Taipei (TW); (75) 3,801.317 A 4, 1974 Tanaka et al. Aloke Kumar, Kolkata (IN); Steven T. 6,911,132 B2 * 6/2005 Pamula et al. ................ 204f600 Wereley, West Lafayette (IN) 6,958,132 B2 10/2005 Chiou et al. 7,508,566 B2 3/2009 Feenstra et al. (73) Assignee: Purdue Research Foundation, West 2006, O146099 A1 7/2006 Wang et al. Lafayette, IN (US) 2006/0165565 A1 7/2006 Ermakov ...................... 422/130 2007/0095669 A1 5/2007 Lau et al. ...................... 204,547 2008.003881.0 A1 2/2008 Pollack et al. (*) Notice: Subject to any disclaimer, the term of this 2009,0170186 A1* 7, 2009 Wu et al. .................... 435/286.1 patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. FOREIGN PATENT DOCUMENTS (21) Appl. No.: 13/380.256 WO WO 2005/OOO970 A1 1, 2005 WO WO 2005/OO1120 A1 1, 2005 PCT Fled: Jun. 25, 2010 WO WO 2005/OO1121 A1 1, 2005 (22) WO WO 2006/094356 A1 9, 2006 (86) PCT NO.: PCT/US2O10/040O31 OTHER PUBLICATIONS S371 (c)(1), G. J. Shah, Electrowetting on dielectric (EWOD) for Biochemical (2), (4) Date: Dec. 22, 2011 Applications: Particle Manipulation for Separation in Droplet (87) PCT Pub. No.: WO2O10/151794 Microfluidics, University of California (2008).* PCT Pub. -

PRELIMINARY PROGRAM (Ver. 4) the 26Th IEEE International
6.12.2012 PRELIMINARY PROGRAM (Ver. 4) The 26th IEEE International Conference on Micro Electro Mechanical Systems IEEE MEMS 2013 January 20 – 24, 2013 Taipei International Convention Center Monday, January 21, 2013 Welcome Address 8:30-9:00 Invited Talk 1 9:00-9:40 Prof. James Liao UCLA, USA 1 BIO-MEMS 9:40-10:40 1-1 (1714) CONTROLLED DELIVERY OF ANTIANGIOGENIC DRUG TO HUMAN EYE TISSUE USING A MEMS DEVICE Fatemeh Nazly Pirmoradi1, Kevin Ou2, John K. Jackson2, Kevin Letchford2, Jing Cui2, Tom Zhao2, Joanne A. Matsubara2, Helen Burt2, Mu Chiao2 Liwei Lin1 1University of California, Berkeley, USA, 2University of British Columbia, Canada 1-2 (1166) QUANTITATIVE EVALUATION OF THE INFLUENCE OF DOPAMINERGIC NEURON ON FLAPPING LOCOMOTION Koki Azuma, Hidetoshi Takahashi, Tetsuo Kan, Jun Tanimura, Kei Ito, Kiyoshi Matsumoto, Isao Shimoyama The University of Tokyo, Japan 1-3 (1627) MEMS STRUCTURE WITH TUNABLE STIFFNESS USING THE MAGNETORHEOLOGICAL EFFECT Guang-Yue Liu, Weileun Fang National Tsing Hua University, Taiwan Break & Exhibition Inspection 10:40-11:10 2 Bio Inspired MEMS 11:10-12:30 2-1 (1821) A SELF-SWIMMING MICROBIAL-ROBOT USING MICROFABRICATED BIOPOLYMER Kazuhiko Higashi1, Tomonori Kano1, Norihisa Miki2 1Keio University, Japan, 2Keio University, JST PRESTO, Japan 2-2 (1489) MUSCLE BASED BIOACTUATOR DRIVEN IN AIR Yuya Morimoto1, Hiroaki Onoe2, Shoji Takeuchi2 1The University of Tokyo, Japan, 2The University of Tokyo, ERATO Takeuchi Biohybrid Project, Japan 2-3 (1704) EFFECTIVENESS OF BRISTLED WING OF THRIPS Ken Sato, Hidetoshi Takahashi, -
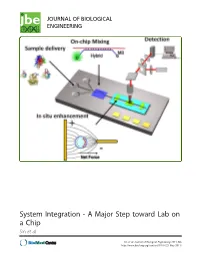
System Integration - a Major Step Toward Lab on a Chip Sin Et Al
System Integration - A Major Step toward Lab on a Chip Sin et al. Sin et al. Journal of Biological Engineering 2011, 5:6 http://www.jbioleng.org/content/5/1/6 (25 May 2011) Sin et al. Journal of Biological Engineering 2011, 5:6 http://www.jbioleng.org/content/5/1/6 REVIEW Open Access System Integration - A Major Step toward Lab on a Chip Mandy LY Sin1, Jian Gao1,2, Joseph C Liao3 and Pak Kin Wong1,4* Abstract Microfluidics holds great promise to revolutionize various areas of biological engineering, such as single cell analysis, environmental monitoring, regenerative medicine, and point-of-care diagnostics. Despite the fact that intensive efforts have been devoted into the field in the past decades, microfluidics has not yet been adopted widely. It is increasingly realized that an effective system integration strategy that is low cost and broadly applicable to various biological engineering situations is required to fully realize the potential of microfluidics. In this article, we review several promising system integration approaches for microfluidics and discuss their advantages, limitations, and applications. Future advancements of these microfluidic strategies will lead toward translational lab-on-a-chip systems for a wide spectrum of biological engineering applications. Background In the past decades, microfluidics has undergone rapid Microfluidics is a multidisciplinary field investigating the development with numerous new fabrication techniques behavior and the manipulation of small amounts of fluids and device designs. There are a large number of publica- with characteristic length scales from nanometers to hun- tions and patents of microfluidic devices functioning as dreds of micrometers [1,2]. -
Monday Program
MONDAY PROGRAM SUNDAY, JANUARY 27 12:00 - Registration Lobby (1F) 18:00 17:00 - Welcome Reception Lobby (1F) 19:00 MONDAY, JANUARY 28 07:00 Registration Lobby (1F) 08:00 Opening Ceremony Grand Ballroom 101~105 (1F) PLENARY PRESENTATION I Session Chair: Shoji Takeuchi, The University of Tokyo, JAPAN Grand Ballroom 101~105 (1F) 08:30 CARDIOMYOCYTES AS HIGH-POWER BUILDING BLOCKS FOR BIO- HYBRID MACHINES Kit Parker Harvard University, USA In an effort to better understand the human heart, our team initiated an effort to identify the fundamental rules of muscular pumps. We reasoned that by reverse engineering marine lifeforms, we would be better able to understand the structure- function relationships in the heart. We reverse engineered life forms such as the jellyfish and sting ray by elucidating how they are built and how they swim. These studies led us to the conclusion that we could use cardiac myocytes as a building block to replicate these marine structure-function relationships in synthetic systems. Furthermore, we decided to use robotics, specifically biohybrid robotics, as an experimental tool to study the fundamental design of muscular pumps. I will present results from these studies. Oral Session I : Micro Robots Session Chair: Franz Lärmer, Bosch, GERMANY Grand Ballroom 101~105 (1F) 09:30 AWARD NOMINEE* DESIGN OF THE FIRST SUB-MILLIGRAM FLAPPING WING AERIAL VEHICLE Palak Bhushan and Claire J. Tomlin University of California, Berkeley, USA Here we report the first sub-milligram flapping wing vehicle which is able to mimic insect wing kinematics. Wing stroke amplitude of 90º and wing pitch amplitude of 80º is demonstrated.