Molecular Phylogeny and Comparative Pollen Morphology of the Genus Hexastylis (Aristolochiaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Wild Ginger, Asarum Spp
A Horticulture Information article from the Wisconsin Master Gardener website, posted 27 June 2005 Wild Ginger, Asarum spp. There are 60-70 species of woodland perennials in the genus Asarum. These great foliage plants in the family Aristolochiaceae make excellent ground covers for shady sites. Their leaves vary considerably in texture, colors of green and patterning. They all need rich organic soil with plenty of moisture to thrive. Under favorable conditions they spread quickly and vigorously. Of these numerous species, European wild ginger, A. europaeum, and wild ginger, A. ca- nadense, are the most commonly available to Asarum europeaum has at- tractive glossy leaves. American gardeners. Both spread slowly to form dense colonies once established. The interest- ing but inconspicuous, dark brown, reddish or purple, bell-shaped fl owers are produced near the ground in spring, hidden by the leaves and blending in with The fl owers of wild gin- soil and leaf litter. ger, Asarum canadense, are small, dark-colored European Wild Ginger (A. europeaum) and hidden by the foliage. This elegant plant with glossy, dark green, nearly rounded leaves makes an excellent ground cover. Plants form neat clumps up to 6 inches high and remain evergreen where winters are not too harsh; in Wisconsin the leaves generally die back to the ground. The leaves are produced in pairs and the small, greenish-brown drooping fl owers are rarely noticed, being hidden by the foliage. This plant prefers part to full shade and rich, moist soil – but has done very well in my garden on clay soil with summer sun until about 2:00 p.m. -

Hexastylis Naniflora)
1 IDENTIFICATION PAGE FY 2012 NCDOT Research Program RESEARCH PROPOSAL Proposal ID 2111 Proposal Title Development of molecular and morphological tools to circumscribe and identify the Dwarf Flowered Heartleaf (Hexastylis naniflora) Period of Performance August 2011-July 2013 Budget by Academic 2011-12 budget = $129,008.00 Year 2012-13 budget = $100,796.00 including Total Total budget = $229,804.00 Department & Biology University Conducting Appalachian State University Work Principal Investigator Zack Murrell Department of Biology Appalachian State University Boone, NC 28608 Phone: 828-262-2674 FAX: 828-262-2127 Email: [email protected] Matt Estep Other Key Researcher(s) Department of Biology Appalachian State University Boone, NC 28608 Jackie Wagner 13 Romney St., Charleston, SC 29403 Joe McKenna Department of Biology Appalachian State University Boone, NC 28608 2 Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. RP 2012-17 …leave blank… …leave blank… 4. Title and Subtitle 5. Report Date Development of molecular and morphological tools to February 15, 2015 circumscribe and identify the Dwarf Flowered Heartleaf (Hexastylis naniflora) 6. Performing Organization Code …leave blank… 7. Author(s) 8. Performing Organization Report No. Zack E. Murrell, Jackie Wagner, Matt Estep …leave blank… 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Department of Biology …leave blank… Appalachian State University Boone, NC 11. Contract or Grant No. …leave blank… 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered North Carolina Department of Transportation Final Report Research and Analysis Group 1 South Wilmington Street Aug 16, 2011-Aug 15, 2014 Raleigh, North Carolina 27601 14. -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies. -

Chestnut Oak Forest (White Pine Subtype)
CHESTNUT OAK FOREST (WHITE PINE SUBTYPE) Concept: The White Pine Subtype encompasses lower elevation Quercus montana forests that have a significant component of Pinus strobus, which may range from a substantial minority to codominant in the canopy. The lower strata in this subtype appear to overlap the less extreme range of variation of the Dry Heath Subtype and the Herb Subtype. Distinguishing Features: Chestnut Oak Forest (White Pine Subtype) is distinguished from all other communities by the combination of Quercus montana with Pinus strobus, without a component of Quercus alba. The White Pine Subtype subtype should only be used where white pine is believed to be naturally present, not for forests where it has been planted or where it likely spread from nearby plantings. Forests with a more mesophytic composition, such as the forests of Quercus rubra and Pinus strobus with Rhododendron maximum that occur around Linville Falls, are treated as the Mesic Subtype. Synonyms: Pinus strobus - Quercus (coccinea, prinus) / (Gaylussacia ursina, Vaccinium stamineum) Forest (CEGL007519). Ecological Systems: Southern Appalachian Oak Forest (CES202.886). Sites: The White Pine Subtype occurs on open slopes and spur ridges. It often is in steep gorges or in the most rugged foothill ranges. Most example are at 1000-2500 feet elevation, but a few are reported upward to 4000 feet or even higher. Soils: Soils are generally Typic Dystrochrepts, especially Chestnut and Edneyville, less often Typic Hapludults such as Tate or Cowee. Hydrology: Sites are dry and well drained but may be somewhat less stressful because of topographic sheltering. Vegetation: The forest canopy is dominated by a mix of Pinus strobus and Quercus montana, occasionally with Quercus coccinea or Quercus rubra codominant. -

Gardenergardener®
Theh American A n GARDENERGARDENER® The Magazine of the AAmerican Horticultural Societyy January / February 2016 New Plants for 2016 Broadleaved Evergreens for Small Gardens The Dwarf Tomato Project Grow Your Own Gourmet Mushrooms contents Volume 95, Number 1 . January / February 2016 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2016 Seed Exchange catalog now available, upcoming travel destinations, registration open for America in Bloom beautifi cation contest, 70th annual Colonial Williamsburg Garden Symposium in April. 11 AHS MEMBERS MAKING A DIFFERENCE Dale Sievert. 40 HOMEGROWN HARVEST Love those leeks! page 400 42 GARDEN SOLUTIONS Understanding mycorrhizal fungi. BOOK REVIEWS page 18 44 The Seed Garden and Rescuing Eden. Special focus: Wild 12 NEW PLANTS FOR 2016 BY CHARLOTTE GERMANE gardening. From annuals and perennials to shrubs, vines, and vegetables, see which of this year’s introductions are worth trying in your garden. 46 GARDENER’S NOTEBOOK Link discovered between soil fungi and monarch 18 THE DWARF TOMATO PROJECT BY CRAIG LEHOULLIER butterfl y health, stinky A worldwide collaborative breeds diminutive plants that produce seeds trick dung beetles into dispersal role, regular-size, fl avorful tomatoes. Mt. Cuba tickseed trial results, researchers unravel how plants can survive extreme drought, grant for nascent public garden in 24 BEST SMALL BROADLEAVED EVERGREENS Delaware, Lady Bird Johnson Wildfl ower BY ANDREW BUNTING Center selects new president and CEO. These small to mid-size selections make a big impact in modest landscapes. 50 GREEN GARAGE Seed-starting products. 30 WEESIE SMITH BY ALLEN BUSH 52 TRAVELER’S GUIDE TO GARDENS Alabama gardener Weesie Smith championed pagepage 3030 Quarryhill Botanical Garden, California. -

Aristolochiaceae – Birthwort Family
ARISTOLOCHIACEAE – BIRTHWORT FAMILY Plant: herbs (perennial) or woody vines, sometimes shrubs, may be aromatic Stem: Root: Leaves: alternate, simple or basal; Large and heart-shaped without teeth, sometimes 3-lobed Flowers: perfect, irregular (zygomorphic); 3 sepals that are reddish-brown, purple or brown that form triangular flowers, or sometimes ‘S’-shaped or pipe- like; no petals; (5)6 or 12 stamens; ovary superior or inferior, 1 pistil, 4-6 carpels, 1 style, 3-6 stigmas; sometimes carrion-scented Fruit: capsules, 4-6 chambered, with seeds Other: wild ginger most common in this area, family most common in the tropics; Dicotyledons Group Genera: 5 genera; locally Hexastylis, Aristolochia, Asarum (wild ginger) WARNING – family descriptions are only a layman’s guide and should not be used as definitive Flower Morphology in the Aristolochiaceae (Birthwort) Family) Genus Asarum Genus Aristolochia Flower pipe-like, climbing stem (woody) Flowers and stem from rhizome, flower regular Woolly [Pipe-Vine] Dutchman's Pipe Wild Ginger ARISTOLOCHIACEAE – BIRTHWORT FAMILY Woolly [Pipe-Vine] Dutchman's Pipe; Aristolochia tomentosa Sims [Canadian] Wild Ginger; Asarum canadense L. Woolly [Pipe-Vine] Dutchman's Pipe USDA Aristolochia tomentosa Sims Aristolochiaceae (Birthwort Family) Alley Springs, Ozark National Scenic Riverways, Shannon County, Missouri Notes: vine, woody at base; calyx (no petals) flower, ‘S’-shaped (like curved pipe), pale greenish yellow, hairy, purple inside; leaves entire, cordate; both stem and leaves downy hairy; fruit a 6- ribbed capsule; late spring to summer [V Max Brown, 2006] [Canadian] Wild Ginger USDA Asarum canadense L. Aristolochiaceae (Birthwort Family) Maumee River Metroparks, Lucas County, Ohio Notes: calyx flower (no petals), 3-lobed, reddish to purple-brown inside, solitary; 2 leaves, heart-shaped with cordate base, long petioles from rhizomes; no stems; root tastes and smells like ginger; spring to summer [V Max Brown, 2004]. -

An Encyclopedia of Shade Perennials This Page Intentionally Left Blank an Encyclopedia of Shade Perennials
An Encyclopedia of Shade Perennials This page intentionally left blank An Encyclopedia of Shade Perennials W. George Schmid Timber Press Portland • Cambridge All photographs are by the author unless otherwise noted. Copyright © 2002 by W. George Schmid. All rights reserved. Published in 2002 by Timber Press, Inc. Timber Press The Haseltine Building 2 Station Road 133 S.W. Second Avenue, Suite 450 Swavesey Portland, Oregon 97204, U.S.A. Cambridge CB4 5QJ, U.K. ISBN 0-88192-549-7 Printed in Hong Kong Library of Congress Cataloging-in-Publication Data Schmid, Wolfram George. An encyclopedia of shade perennials / W. George Schmid. p. cm. ISBN 0-88192-549-7 1. Perennials—Encyclopedias. 2. Shade-tolerant plants—Encyclopedias. I. Title. SB434 .S297 2002 635.9′32′03—dc21 2002020456 I dedicate this book to the greatest treasure in my life, my family: Hildegarde, my wife, friend, and supporter for over half a century, and my children, Michael, Henry, Hildegarde, Wilhelmina, and Siegfried, who with their mates have given us ten grandchildren whose eyes not only see but also appreciate nature’s riches. Their combined love and encouragement made this book possible. This page intentionally left blank Contents Foreword by Allan M. Armitage 9 Acknowledgments 10 Part 1. The Shady Garden 11 1. A Personal Outlook 13 2. Fated Shade 17 3. Practical Thoughts 27 4. Plants Assigned 45 Part 2. Perennials for the Shady Garden A–Z 55 Plant Sources 339 U.S. Department of Agriculture Hardiness Zone Map 342 Index of Plant Names 343 Color photographs follow page 176 7 This page intentionally left blank Foreword As I read George Schmid’s book, I am reminded that all gardeners are kindred in spirit and that— regardless of their roots or knowledge—the gardening they do and the gardens they create are always personal. -
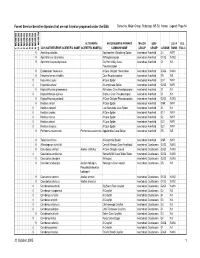
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Cumberland Gap National Historic Park
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Natural Resource Condition Assessment for Cumberland Gap National Historical Park Natural Resource Report NPS/CUGA/NRR—2013/620 ON THE COVER Pinnacle Overlook Photograph by D. McPherson Natural Resource Condition Assessment for Cumberland Gap National Historical Park Natural Resource Report NPS/CUGA/NRR—2013/620 Gary Sundin, Luke Worsham, Nathan P. Nibbelink, Michael T. Mengak, Gary Grossman Warnell School of Forestry and Natural Resources University of Georgia 180 E. Green St. Athens, GA 30602 January 2013 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. This report received formal peer review by subject-matter experts who were not directly involved in the collection, analysis, or reporting of the data, and whose background and expertise put them on par technically and scientifically with the authors of the information. -

Chamaelirium Luteum (L.) A
New England Plant Conservation Program Chamaelirium luteum (L.) A. Gray Devil's Bit Conservation and Research Plan for New England Prepared by: Dorothy J. Allard, Ph.D. Analytical Resources, L.L.C. P. O. Box 279 East Montpelier, VT 05651 For: New England Wild Flower Society 180 Hemenway Road Framingham, MA 01701 508/877-7630 e-mail: [email protected] • website: www.newfs.org Approved, Regional Advisory Council, May 2003 SUMMARY Chamaelirium luteum (L.) A. Gray is a dioecious perennial in the Liliaceae, and is the only species in its genus. It occurs in 24 eastern states and was once known from southern Ontario. It is common near the center of its range, but rare on its western and northern boundaries. In New England, it occurs in Connecticut and Massachusetts, where it is at the northern edge of its range. At one time more commonplace, now only 11 populations in the two states remain, and several of these have very few individuals. Chamaelirium luteum has a basal rosette of leaves and a single flowering stalk with either male or female flowers. Flowers are white or greenish white; male flowers fade to yellow. Occasional plants can be found with a few perfect flowers at the base of the male inflorescence, a condition called polygamo-monoecy. Although it has a wide habitat tolerance, Chamaelirium luteum typically grows on slopes of any aspect in open, mesic, rich hardwood forests, or in wet meadows. It requires partially open conditions in order to flower, but persists for years as vegetative rosettes in more shaded situations. -

Biological Evalutation for The
BIOLOGICAL EVALUTATION FOR THE CRAWLEY BRANCH SOUTHERN YELLOW PINE RESTORATION PROJECT PISGAH NATIONAL FOREST GRANDFATHER RANGER DISTRICT CALDWELL COUNTY, NC Contact Person: Christopher L. Williams Pisgah Zone Wildlife Biologist Grandfather RD Nebo, NC 28761 (828) 652-2144 email: [email protected] July 10, 2017 ABSTRACT: Based on the findings contained within this biological evaluation, the Crawley Branch southern yellow pine restoration project proposal will not affect any proposed, threatened, or endangered, aquatic, botanical, or terrestrial wildlife species, or habitat. Consultation with the US Fish and Wildlife Service is not required. I. INTRODUCTION A. Biological Evaluation/Assessment As a result of a recent court decision, Forest Plan Amendment #14 and the Region 8 Supplement to Forest Service Manual 2670 are no longer in effect. This Biological Evaluation (BE) for the Crawley Branch southern yellow pine restoration project follows the process used to decide when to inventory for federally proposed, threatened, endangered, or Regional Forester’s sensitive species (PETS) that is consistent with the requirement found in the Vegetation Management EIS for the Appalachian Mountains. B. Purpose and Format The purpose of this BE is to provide the decision maker with relevant biological information as to the possible effects and impacts this proposal may have to PETS. This BE documents the possible biological effects and impacts of the proposed action activities known as the Crawley Branch southern yellow pine restoration project. A detailed description of the proposal is disclosed in the Decision Memo. A list of project design features and monitoring is disclosed in the Decision Memo as well. A list of definitions, including analysis areas is located at the end of this BE.