Circular 14. English and Local Common Names of Philippine Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Present Status of Fish Biodiversity and Abundance in Shiba River, Bangladesh
Univ. J. zool. Rajshahi. Univ. Vol. 35, 2016, pp. 7-15 ISSN 1023-6104 http://journals.sfu.ca/bd/index.php/UJZRU © Rajshahi University Zoological Society Present status of fish biodiversity and abundance in Shiba river, Bangladesh D.A. Khanom, T Khatun, M.A.S. Jewel*, M.D. Hossain and M.M. Rahman Department of Fisheries, University of Rajshahi, Rajshahi 6205, Bangladesh Abstract: The study was conducted to investigate the abundance and present status of fish biodiversity in the Shiba river at Tanore Upazila of Rajshahi district, Bangladesh. The study was conducted from November, 2016 to February, 2017. A total of 30 species of fishes were recorded belonging to nine orders, 15 families and 26 genera. Cypriniformes and Siluriformes were the most diversified groups in terms of species. Among 30 species, nine species under the order Cypriniformes, nine species of Siluriformes, five species of Perciformes, two species of Channiformes, two species of Mastacembeliformes, one species of Beloniformes, one species of Clupeiformes, one species of Osteoglossiformes and one species of Decapoda, Crustacea were found. Machrobrachium lamarrei of the family Palaemonidae under Decapoda order was the most dominant species contributing 26.29% of the total catch. In the Shiba river only 6.65% threatened fish species were found, and among them 1.57% were endangered and 4.96% were vulnerable. The mean values of Shannon-Weaver diversity (H), Margalef’s richness (D) and Pielou’s (e) evenness were found as 1.86, 2.22 and 0.74, respectively. Relationship between Shannon-Weaver diversity index (H) and pollution indicates the river as light to moderate polluted. -

DYNAMIC HABITAT USE of ALBACORE and THEIR PRIMARY PREY SPECIES in the CALIFORNIA CURRENT SYSTEM Calcofi Rep., Vol
MUHLING ET AL.: DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM CalCOFI Rep., Vol. 60, 2019 DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM BARBARA MUHLING, STEPHANIE BRODIE, MICHAEL JACOX OWYN SNODGRASS, DESIREE TOMMASI NOAA Earth System Research Laboratory University of California, Santa Cruz Boulder, CO Institute for Marine Science Santa Cruz, CA CHRISTOPHER A. EDWARDS ph: (858) 546-7197 Ocean Sciences Department [email protected] University of California, Santa Cruz, CA BARBARA MUHLING, OWYN SNODGRASS, YI XU HEIDI DEWAR, DESIREE TOMMASI, JOHN CHILDERS Department of Fisheries and Oceans NOAA Southwest Fisheries Science Center Delta, British Columbia, Canada San Diego, CA STEPHANIE SNYDER STEPHANIE BRODIE, MICHAEL JACOX Thomas More University, NOAA Southwest Fisheries Science Center Crestview Hills, KY Monterey, CA ABSTRACT peiods, krill, and some cephalopods (Smith et al. 2011). Juvenile north Pacific albacore Thunnus( alalunga) for- Many of these forage species are fished commercially, age in the California Current System (CCS), supporting but also support higher-order predators further up the fisheries between Baja California and British Columbia. food chain, such as other exploited species (e.g., tunas, Within the CCS, their distribution, abundance, and for- billfish) and protected resources (e.g., marine mammals aging behaviors are strongly variable interannually. Here, and seabirds) (Pikitch et al. 2004; Link and Browman we use catch logbook data and trawl survey records to 2014). Effectively managing marine ecosystems to pre- investigate how juvenile albacore in the CCS use their serve these trophic linkages, and improve robustness of oceanographic environment, and how their distributions management strategies to environmental variability, thus overlap with the habitats of four key forage species. -
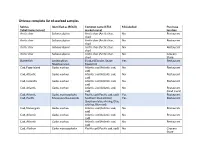
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

Daily Ration of Japanese Spanish Mackerel Scomberomorus Niphonius Larvae
FISHERIES SCIENCE 2001; 67: 238–245 Original Article Daily ration of Japanese Spanish mackerel Scomberomorus niphonius larvae J SHOJI,1,* T MAEHARA,2,a M AOYAMA,1 H FUJIMOTO,3 A IWAMOTO3 AND M TANAKA1 1Laboratory of Marine Stock-enhancement Biology, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Sakyo, Kyoto 606-8502, 2Toyo Branch, Ehime Prefecture Chuyo Fisheries Experimental Station, Toyo, Ehime 799-1303, and 3Yashima Station, Japan Sea-farming Association, Takamatsu, Kagawa 761-0111, Japan SUMMARY: Diel successive samplings of Japanese Spanish mackerel Scomberomorus niphonius larvae were conducted throughout 24 h both in the sea and in captivity in order to estimate their daily ration. Using the Elliott and Persson model, the instantaneous gastric evacuation rate was estimated from the depletion of stomach contents (% dry bodyweight) with time during the night for wild fish (3.0–11.5 mm standard length) and from starvation experiments for reared fish (8, 10, and 15 days after hatching (DAH)). Japanese Spanish mackerel is a daylight feeder and exhibited piscivorous habits from first feeding both in the sea and in captivity. Feeding activity peaked at dusk. The esti- mated daily ration for wild larvae were 111.1 and 127.2% in 1996 and 1997, respectively; and those for reared larvae ranged from 90.6 to 111.7% of dry bodyweight. Based on the estimated value of daily rations for reared fish, the total number of newly hatched red sea bream Pagrus major larvae preyed by a Japanese Spanish mackerel from first feeding (5 DAH) to beginning of juvenile stage (20 DAH) in captivity was calculated to be 1139–1404. -

Size Composition, Growth, Mortality and Yield of Alectis Alexandrinus (Geoffory Saint-Hilaire) in Bonny River, Niger Delta, Nigeria
African Journal of Biotechnology Vol. 8 (23), pp. 6721-6723, 1 December, 2009 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2009 Academic Journals Short Communication Size composition, growth, mortality and yield of Alectis alexandrinus (Geoffory Saint-Hilaire) in Bonny River, Niger Delta, Nigeria S. N. Deekae1, K. O. Chukwu1 and A. J. Gbulubo2 1Department of Fisheries and Aquatic Environment, Rivers State University of Science and Technology, Port-Harcourt Rivers State, Nigeria. 2African Regional Aquaculture Centre, Port Harcourt, Nigeria. Institute for Oceanography and Marine Research, P.M.B.5122, Port Harcourt, Nigeria. Accepted 3 November, 2008 A twelve month study on the size composition, growth, mortality and yield of Alectis alexandrinus revealed a length range of 11.5 - 33.8 cm (standard length). Employing the length frequency method in the FISAT II package gave the following results for the Von Bertanlanffy growth parameters: L = 35.23, K = 0.680, to = 0.3214 and = 2.926. The total mortality (Z) was 2.47, natural mortality (M) 1.39 and fishing mortality (F) 1.08.The relative biomass per recruit (knife edge selection) was Lc/ L = 0.05, E10 = 0.355 and E50 = 0.278. Although the exploitation rate (E) was 0.44 the Emax was 0.421 indicating moderate exploitation of the fish in Bonny River. There is room for increased effort in the fisheries. Key word: Size, growth, mortality, yield, Alectis alexandrinus. INTRODUCTION Alectics alexandrinus is one of the commercially valuable tions or species into categories of high, medium low and fishes in the gulf of Guinea, which could be relevant in very low resilience or productivity have been suggested sport fishing as obtainable in places like Hawaii (Pamela et al., 2001; Musick, 1999). -

Odia: Dhudhiya Magara / Sorrah Magara / Haladia Magara
FISH AND SHELLFISH DIVERSITY AND ITS SUSTAINABLE MANAGEMENT IN CHILIKA LAKE V. R. Suresh, S. K. Mohanty, R. K. Manna, K. S. Bhatta M. Mukherjee, S. K. Karna, A. P. Sharma, B. K. Das A. K. Pattnaik, Susanta Nanda & S. Lenka 2018 ICAR- Central Inland Fisheries Research Institute Barrackpore, Kolkata - 700 120 (India) & Chilika Development Authority C- 11, BJB Nagar, Bhubaneswar- 751 014 (India) FISH AND SHELLFISH DIVERSITY AND ITS SUSTAINABLE MANAGEMENT IN CHILIKA LAKE V. R. Suresh, S. K. Mohanty, R. K. Manna, K. S. Bhatta, M. Mukherjee, S. K. Karna, A. P. Sharma, B. K. Das, A. K. Pattnaik, Susanta Nanda & S. Lenka Photo editing: Sujit Choudhury and Manavendra Roy ISBN: 978-81-938914-0-7 Citation: Suresh, et al. 2018. Fish and shellfish diversity and its sustainable management in Chilika lake, ICAR- Central Inland Fisheries Research Institute, Barrackpore, Kolkata and Chilika Development Authority, Bhubaneswar. 376p. Copyright: © 2018. ICAR-Central Inland Fisheries Research Institute (CIFRI), Barrackpore, Kolkata and Chilika Development Authority, C-11, BJB Nagar, Bhubaneswar. Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission from the copyright holders. Photo credits: Sujit Choudhury, Manavendra Roy, S. K. Mohanty, R. K. Manna, V. R. Suresh, S. K. Karna, M. Mukherjee and Abdul Rasid Published by: Chief Executive Chilika Development Authority C-11, BJB Nagar, Bhubaneswar-751 014 (Odisha) Cover design by: S. K. Mohanty Designed and printed by: S J Technotrade Pvt. -

Simulations of Fishing Effects on the Southern Benguela Fish Community Using an Individual-Based Model: Learning from a Comparison with Ecosim
Ecosystem Approaches to Fisheries in the Southern Benguela Afr. J. mar. Sci. 26: 95–114 2004 95 SIMULATIONS OF FISHING EFFECTS ON THE SOUTHERN BENGUELA FISH COMMUNITY USING AN INDIVIDUAL-BASED MODEL: LEARNING FROM A COMPARISON WITH ECOSIM Y-J. SHIN*, L. J. SHANNON† and P. M. CURY* By applying an individual-based model (OSMOSE) to the southern Benguela ecosystem, a multispecies analysis is proposed, complementary to that provided by the application of ECOPATH/ECOSIM models. To reconstruct marine foodwebs, OSMOSE is based on the hypothesis that predation is a size-structured process. In all, 12 fish species, chosen for their importance in terms of biomass and catches, are explicitly modelled. Growth, repro- duction and mortality parameters are required to model their dynamics and trophic interactions. Maps of mean spatial distribution of the species are compiled from published literature. Taking into account the spatial component is necessary because spatial co-occurrence determines potential interactions between predatory fish and prey fish of suitable size. To explore ecosystem effects of fishing, different fishing scenarios, previously examined using ECOSIM, are simulated using the OSMOSE model. They explore the effects of targeting fish species in the southern Benguela considered to be predators (Cape hake Merluccius capensis and M. paradoxus) or prey (anchovy Engraulis encrasicolus, sardine Sardinops sagax, round herring Etrumeus whiteheadi). Simulation results are compared and are generally consistent with those obtained using an ECOSIM model. This cross-validation appears to be a promising means of evaluating the robustness of model outputs, when separate validation of marine ecosystem models are still difficult to perform. -

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

Predator/Prey Interactions, Competition, Multi
Current understanding of trophic dynamics In the Mid-Atlantic Bluefish diets, Mid Atlantic and Southern New England, NEFSC surveys 100% All others All Flatfish 90% Unid fish Unid Unid fish Unid fish Unid fish Scup 80% Scup Unid fish Scup Bluefish Butterfish Bluefish 70% Mackerel Bluefish Butterfish Butterfish Hakes 60% Loligo Drums Butterfish Unid Squid Ocean pout Round herring 50% Butterfish Ctenophores Menhaden Loligo Loligo Loligo 40% Loligo Unid Squid Round herring Illex Bay anchovy Unid Squid Unid Squid Silver anchovy Round herring Round herring 30% Bay anchovy Menhaden Unid herring Bay anchovy Menhaden Bay anchovy Striped anchovy 20% Silver anchovy Unid anchovies Bay anchovy Striped anchovy Unid anchovies 10% Striped anchovy Unid anchovies Sand lances Unid anchovies Sand lances Sand lances Sand lances 0% 1977-86 1987-96 1997-2006 2007-2013 Food web models partition mortality 100% 90% 80% If Pred > F, 70% Pred Re-evaluate 60% constant M 50% 40% Proportion of total Mortality total Proportion of F 30% 20% 10% Proportion of total mortality total of Proportion 0% Longnose skate P. Halibut W. Pollock Squids Gaichas et al. 2010 Fishing Predation Other Link et al. 2008. The Northeast U.S. continental shelf Energy Modeling and Analysis exercise (EMAX): Ecological network model development and basic ecosystem metrics. Journal of Marine Systems 74: 453-474 Intermediate tactical multispecies models An intermediate-complexity tactical ecosystem assessment tool combines: Standard stock assessment Ecosystem considerations • Structured population -

Invasive Catfish Management Strategy August 2020
Invasive Catfish Management Strategy August 2020 A team from the Virginia Department of Game and Inland Fisheries uses electrofishing to monitor invasive blue catfish in the James River in 2011. (Photo by Matt Rath/Chesapeake Bay Program) I. Introduction This management strategy portrays the outcomes of an interactive workshop (2020 Invasive Catfish Workshop) held by the Invasive Catfish Workgroup at the Virginia Commonwealth University (VCU) Rice Rivers Center in Charles City, Virginia on January 29-30, 2020. The workshop convened a diverse group of stakeholders to share the current scientific understanding and priority issues associated with invasive catfishes in Chesapeake Bay. The perspectives shared and insights gained from the workshop were used to develop practical, synergistic recommendations that will improve management and mitigate impacts of these species across jurisdictions within the watershed. Blue catfish (Ictalurus furcatus) and flathead catfish (Pylodictis olivaris) are native to the Ohio, Missouri, Mississippi, and Rio Grande river basins, and were introduced into the Virginia tributaries of Chesapeake Bay in the 1960s and 1970s to establish a recreational fishery. These non-native species have since spread, inhabiting nearly all major tributaries of the Bay watershed. Rapid range expansion and population growth, particularly of blue catfish, have led to increasing concerns about impacts on the ecology of the Chesapeake Bay ecosystem. 1 Chesapeake Bay Management Strategy Invasive Catfish Blue and flathead catfishes are long-lived species that can negatively impact native species in Chesapeake Bay through predation and resource competition. Blue catfish are generalist feeders that prey on a wide variety of species that are locally abundant, including those of economic importance and conservation concern, such as blue crabs, alosines, Atlantic menhaden, American eels, and bay anchovy. -

126-1^2 on SOME INTERESTING and NEW RECORDS of MARINE FISHES from INDIA* Central Marine F
i. Mar. biol Ass. tndia, 1968, 10 (1): 126-1^2 ON SOME INTERESTING AND NEW RECORDS OF MARINE FISHES FROM INDIA* By V. SRIRAMACHANDRA MURTY Central Marine Fisheries Research Institute, Mandapam Camp WHILE examining the fish landings by shore seines and trawl nets at various fishing centres along the Palk Bay and Gulf of Mannar in the vicinity of Mandapam the author came across several specimens of Drepane longimana (Bloch and Schneider) which is little known and Drepane punctata (Linnaeus) which was recognised as the only valid species of the genus Drepane, A study of these specimens has shown that these two species are distinct as shown by some authors (vide Text). A brief com parative account of these two species is given in this paper, along with a few remarks and key to distinguish the two species. The author has also been able to collect specimens of Platycephalus isacanthus Cuvier from the above catches, and a single specimen of Stethojulis interrupta (Bleeker) from the inshore waters of Gulf of Mannar caught in dragnet, whose occurrence, in Indian seas, is so far not known. Brief descriptions of these two species are also given in this paper. Family; DREPANIDAE Drepane longimana (Bloch and Schneider) This species was first described by its authors from Tranquebar. Cuvier and Valenciennes (1831) studied the specimens of the genus Drepane, both morphologi cally and anatomically and distinguished the two species D. longimana (Bloch and Schneider) and D. punctata (Linnaeus). Cantor (1850) also recognised the two species as valid. But subsequently Gunther (1860), Bleeker (1877) and Day (1878) recognised D. -

Fisheries of the Northeast
FISHERIES OF THE NORTHEAST AMERICAN BLUE LOBSTER BILLFISHES ATLANTIC COD MUSSEL (Blue marlin, Sailfish, BLACK SEA BASS Swordfish, White marlin) CLAMS DRUMS BUTTERFISH (Arc blood clam, Arctic surf clam, COBIA Atlantic razor clam, Atlantic surf clam, (Atlantic croaker, Black drum, BLUEFISH (Gulf butterfish, Northern Northern kingfish, Red drum, Northern quahog, Ocean quahog, harvestfish) CRABS Silver sea trout, Southern kingfish, Soft-shelled clam, Stout razor clam) (Atlantic rock crab, Blue crab, Spot, Spotted seatrout, Weakfish) Deep-sea red crab, Green crab, Horseshoe crab, Jonah crab, Lady crab, Northern stone crab) GREEN SEA FLATFISH URCHIN EELS (Atlantic halibut, American plaice, GRAY TRIGGERFISH HADDOCK (American eel, Fourspot flounder, Greenland halibut, Conger eel) Hogchoker, Southern flounder, Summer GROUPERS flounder, Winter flounder, Witch flounder, (Black grouper, Yellowtail flounder) Snowy grouper) MACKERELS (Atlantic chub mackerel, MONKFISH HAKES JACKS Atlantic mackerel, Bullet mackerel, King mackerel, (Offshore hake, Red hake, (Almaco jack, Amberjack, Bar Silver hake, Spotted hake, HERRINGS jack, Blue runner, Crevalle jack, Spanish mackerel) White hake) (Alewife, Atlantic menhaden, Atlantic Florida pompano) MAHI MAHI herring, Atlantic thread herring, Blueback herring, Gizzard shad, Hickory shad, Round herring) MULLETS PORGIES SCALLOPS (Striped mullet, White mullet) POLLOCK (Jolthead porgy, Red porgy, (Atlantic sea Scup, Sheepshead porgy) REDFISH scallop, Bay (Acadian redfish, scallop) Blackbelly rosefish) OPAH SEAWEEDS (Bladder