Evolutionary Ecology of Giraffes (Giraffa Camelopardalis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ballistic Protective Properties of Material Representative of English Civil War Buff-Coats and Clothing
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UWE Bristol Research Repository International Journal of Legal Medicine (2020) 134:1949–1956 https://doi.org/10.1007/s00414-020-02378-x ORIGINAL ARTICLE Ballistic protective properties of material representative of English civil war buff-coats and clothing Brian May1 & Richard Critchley1 & Debra Carr1,2 & Alan Peare1 & Keith Dowen3 Received: 19 March 2020 /Accepted: 15 July 2020 / Published online: 21 July 2020 # The Author(s) 2020 Abstract One type of clothing system used in the English Civil War, more common amongst cavalrymen than infantrymen, was the linen shirt, wool waistcoat and buff-coat. Ballistic testing was conducted to estimate the velocity at which 50% of 12-bore lead spherical projectiles (V50) would be expected to perforate this clothing system when mounted on gelatine (a tissue simulant used in wound ballistic studies). An estimated six-shot V50 for the clothing system was calculated as 102 m/s. The distance at which the projectile would have decelerated from the muzzle of the weapon to this velocity in free flight was triple the recognised effective range of weapons of the era suggesting that the clothing system would provide limited protection for the wearer. The estimated V50 was also compared with recorded bounce-and-roll data; this suggested that the clothing system could provide some protection to the wearer from ricochets. Finally, potential wounding behind the clothing system was investigated; the results compared favourably with seventeenth century medical writings. Keywords Leather . Linen . Wool . Behind armour blunt trauma . -

Angolan Giraffe (Giraffa Camelopardalis Ssp
Angolan Giraffe (Giraffa camelopardalis ssp. angolensis) Appendix 1: Historical and recent geographic range and population of Angolan Giraffe G. c. angolensis Geographic Range ANGOLA Historical range in Angola Giraffe formerly occurred in the mopane and acacia savannas of southern Angola (East 1999). According to Crawford-Cabral and Verissimo (2005), the historic distribution of the species presented a discontinuous range with two, reputedly separated, populations. The western-most population extended from the upper course of the Curoca River through Otchinjau to the banks of the Kunene (synonymous Cunene) River, and through Cuamato and the Mupa area further north (Crawford-Cabral and Verissimo 2005, Dagg 1962). The intention of protecting this western population of G. c. angolensis, led to the proclamation of Mupa National Park (Crawford-Cabral and Verissimo 2005, P. Vaz Pinto pers. comm.). The eastern population occurred between the Cuito and Cuando Rivers, with larger numbers of records from the southeast corner of the former Mucusso Game Reserve (Crawford-Cabral and Verissimo 2005, Dagg 1962). By the late 1990s Giraffe were assumed to be extinct in Angola (East 1999). According to Kuedikuenda and Xavier (2009), a small population of Angolan Giraffe may still occur in Mupa National Park; however, no census data exist to substantiate this claim. As the Park was ravaged by poachers and refugees, it was generally accepted that Giraffe were locally extinct until recent re-introductions into southern Angola from Namibia (Kissama Foundation 2015, East 1999, P. Vaz Pinto pers. comm.). BOTSWANA Current range in Botswana Recent genetic analyses have revealed that the population of Giraffe in the Central Kalahari and Khutse Game Reserves in central Botswana is from the subspecies G. -

An Antifungal Property of Crude Plant Extracts from Anogeissus Leiocarpus and Terminalia Avicennioides
34 Tanzania Journal of Health Research (2008), Vol. 10, No. 1 An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides A. MANN*, A. BANSO and L.C. CLIFFORD Department of Science Laboratory Technology, The Federal Polytechnic, P.M.B. 55, Bida, Niger State, Nigeria Abstract: Chloroform, ethanolic, methanolic, ethyl acetate and aqueous root extracts of Anogeissus leiocarpus and Ter- minalia avicennioides were investigated in vitro for antifungal activities against Aspergillus niger, Aspergillus fumigatus, Penicillium species, Microsporum audouinii and Trichophyton rubrum using radial growth technique. The plant extracts inhibited the growth of all the test organisms. The minimum inhibitory concentration (MIC) of the extracts ranged between 0.03μg/ml and 0.07μg/ml while the minimum fungicidal concentration ranged between 0.04μg/ml and 0.08μg/ml. Ano- geissus leiocarpus appears to be more effective as an antifungal agent than Terminalia avicennioides. Ethanolic extracts of the two plant roots were more effective than the methanolic, chloroform, or aqueous extracts against all the test fungi. Key words: Anogeissus leiocarpus, Terminalia avicennioides, extracts, inhibitory, fungicidal, Nigeria Introduction sential oil of Aframomum melagulatus fruit inhibited the mycelia growth of Trichophyton mentagrophytes Many of the plant materials used in traditional medi- at a pronounced rate but the inhibitory concentration cine are readily available in rural areas and this has obtained against Aspergillus niger was lower. made traditional medicine relatively cheaper than Since medicinal plants play a paramount role in modern medicine (Apulu et al., 1994). Over Sixty the management of various ailments in rural commu- percent of Nigeria rural population depends on tra- nities, there is therefore, a need for scientific verifica- ditional medicine for their healthcare need (Apulu et tion of their activities against fungi. -

Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout Has in THIS ISSUE Dbeen Awarded the President’S Cup Letter from the President
DSC NEWSLETTER VOLUME 32,Camp ISSUE 5 TalkJUNE 2019 Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout has IN THIS ISSUE Dbeen awarded the President’s Cup Letter from the President .....................1 for her pending world record East African DSC Foundation .....................................2 Defassa Waterbuck. Awards Night Results ...........................4 DSC’s April Monthly Meeting brings Industry News ........................................8 members together to celebrate the annual Chapter News .........................................9 Trophy and Photo Award presentation. Capstick Award ....................................10 This year, there were over 150 entries for Dove Hunt ..............................................12 the Trophy Awards, spanning 22 countries Obituary ..................................................14 and almost 100 different species. Membership Drive ...............................14 As photos of all the entries played Kid Fish ....................................................16 during cocktail hour, the room was Wine Pairing Dinner ............................16 abuzz with stories of all the incredible Traveler’s Advisory ..............................17 adventures experienced – ibex in Spain, Hotel Block for Heritage ....................19 scenic helicopter rides over the Northwest Big Bore Shoot .....................................20 Territories, puku in Zambia. CIC International Conference ..........22 In determining the winners, the judges DSC Publications Update -

Pinjarra Park Printable Form Guide
FREE printable form guides from www.punters.com.au Pinjarra Park Tuesday 25th April 2017 Race 1 PINJARRA RSL MAIDEN 1600m 1:09 pm Race 2 SOUND TELEGRAPH HANDICAP 1500m 1:44 pm Race 3 AURORA LANDSCAPING MAIDEN 1200m 2:19 pm Race 4 PINJARRA REAL ESTATE MAIDEN 1200m 2:54 pm Race 5 WORK CLOBBER MANDURAH MAIDEN 1000m 3:29 pm Race 6 SIGN STRATEGY HANDICAP 1000m 4:04 pm Race 7 WAROONA RURAL SERVICES HANDICAP 2000m 4:34 pm Race 8 RENEW IPL HANDICAP 1300m 5:10 pm Produced for free by Punters.com.au, thanks to William Hill Punters.com.au is your ultimate racing website. Social networking, free form guides, odds comparison, betting deals, the latest news, photos and a revolutionary tipping system allowing punters to buy and sell their horse racing tips. Visit www.punters.com.au for more information. © 2017 Punters Paradise Pty Ltd. If you're reading this copyright notice you're probably thinking of printing lots of copies. Go for it. Give a copy to your mates, your mum and some strangers at the TAB. We want people to have our free form guides. Just don't sell them, alter, change or reproduce parts of this form guide as it's strictly prohibited. While Punters.com.au takes all care in the preparation of information we accept no responsibility nor warrants the accuracy of the information displayed. © 2017 Racing Australia Pty Ltd (RA) (and other parties working with it). Racing materials, including fields, form and results are subject to copyright which is owned by RA and other parties working with it. -
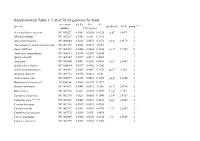
Supplementary Table 1: List of 76 Mt Genomes for Birds
Supplementary Table 1: List of 76 mt genomes for birds. accession Ka/Ks Ka Ks species speed(m/s) Ln S group * * number 13 mt genes Acrocephalus scirpaceus NC 010227 0.0464 0.0288 0.6228 6.6d 1.8871 2 Alectura lathami NC 007227 0.0343 0.032 0.9311 2 Anas platyrhynchos NC 009684 0.0252 0.0073 0.2873 18.5a 2.9178 2 Anomalopteryx didiformis (die out) NC 002779 0.0508 0.0027 0.054 1 Anser albifrons NC 004539 0.0468 0.0088 0.1856 16.1a 2.7788 2 Anseranas semipalmata NC 005933 0.0364 0.0207 0.5628 2 Apteryx haastii NC 002782 0.0599 0.0273 0.4561 1 Apus apus NC 008540 0.0401 0.0241 0.6431 10.6a 2.3609 2 Archilochus colubris NC 010094 0.0397 0.0382 0.9242 2 Ardea novaehollandiae NC 008551 0.0423 0.0067 0.1552 10.2c# 2.322 2 Arenaria interpres NC 003712 0.0378 0.0213 0.581 2 Aythya americana NC 000877 0.0309 0.0092 0.2987 24.4b 3.1946 2 Bambusicola thoracica* EU165706 0.0503 0.0144 0.2872 1 Branta canadensis NC 007011 0.0444 0.0092 0.206 16.7a 2.8154 2 Buteo buteo NC 003128 0.049 0.0167 0.3337 11.6a 2.451 2 Casuarius casuarius NC 002778 0.028 0.0085 0.3048 13.9f 2.6319 2 Cathartes aura * * * * NC 007628 0.0468 0.0239 0.4676 10.6c 2.3609 2 Ciconia boyciana NC 002196 0.0539 0.0031 0.0563 2 Ciconia ciconia NC 002197 0.0501 0.0029 0.0572 9.1d 2.2083 2 Cnemotriccus fuscatus NC 007975 0.0369 0.038 1.0476 2 Corvus frugilegus NC 002069 0.0428 0.0298 0.6526 13a 2.5649 2 Coturnix chinensis NC 004575 0.0325 0.0122 0.3762 2 Coturnix japonica NC 003408 0.0432 0.0128 0.2962 2 Cygnus columbianus NC 007691 0.0327 0.0089 0.2702 18.5a 2.9178 2 Dinornis giganteus -

Human Mating Strategies Human Mating Strategies
Human Mating Strategies Human Mating Strategies As descendants of a long line of successful maters, modern humans have inherited the mating strategies that led to their forebear’s success. These include long-term mating, short-term mating, and mixed mating strategies. This article presents empirical evi- dence supporting evolution-based hypotheses about the complexities of these mating strategies, which differ substantially for men and women. array of adaptations specifically dedicated to the David M. Buss, Professor, task of mating. Department of Psychology, Nowhere do people have an equal desire to mate University of Texas, Austin with all people. Everywhere, some people are pre- ferred as mates, others shunned. Desires are central to all facets of mating. They determine who we are attracted to, and who is attracted to us. They influ- ence which attraction tactics will be successful (those that fulfill desires) and which attraction tac- tics will fail (those that violate desires). Successful mate retention tactics involve continuing to provide resources that fulfill the desires of a mate. Failure to Perhaps no adaptive domain is more central to re- fulfill these desires causes breakup and divorce. At production than mating. Those in our evolutionary every step of the mating process, from mate selec- past who failed to mate failed to become ancestors. tion to mate expulsion, desires determine the Modern humans are all descendants of a long and ground rules. unbroken line of ancestors who succeeded in the complex and sometimes circuitous tasks involved in Sexual Selection and Parental Investment mating. As their descendants, modern humans have Although Charles Darwin (1859) recognized that inherited the adaptations that led to the success of survival was central to the evolutionary process, their ancestors. -

Naming the Extrasolar Planets
Naming the extrasolar planets W. Lyra Max Planck Institute for Astronomy, K¨onigstuhl 17, 69177, Heidelberg, Germany [email protected] Abstract and OGLE-TR-182 b, which does not help educators convey the message that these planets are quite similar to Jupiter. Extrasolar planets are not named and are referred to only In stark contrast, the sentence“planet Apollo is a gas giant by their assigned scientific designation. The reason given like Jupiter” is heavily - yet invisibly - coated with Coper- by the IAU to not name the planets is that it is consid- nicanism. ered impractical as planets are expected to be common. I One reason given by the IAU for not considering naming advance some reasons as to why this logic is flawed, and sug- the extrasolar planets is that it is a task deemed impractical. gest names for the 403 extrasolar planet candidates known One source is quoted as having said “if planets are found to as of Oct 2009. The names follow a scheme of association occur very frequently in the Universe, a system of individual with the constellation that the host star pertains to, and names for planets might well rapidly be found equally im- therefore are mostly drawn from Roman-Greek mythology. practicable as it is for stars, as planet discoveries progress.” Other mythologies may also be used given that a suitable 1. This leads to a second argument. It is indeed impractical association is established. to name all stars. But some stars are named nonetheless. In fact, all other classes of astronomical bodies are named. -

In China: Phylogeny, Host Range, and Pathogenicity
Persoonia 45, 2020: 101–131 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2020.45.04 Cryphonectriaceae on Myrtales in China: phylogeny, host range, and pathogenicity W. Wang1,2, G.Q. Li1, Q.L. Liu1, S.F. Chen1,2 Key words Abstract Plantation-grown Eucalyptus (Myrtaceae) and other trees residing in the Myrtales have been widely planted in southern China. These fungal pathogens include species of Cryphonectriaceae that are well-known to cause stem Eucalyptus and branch canker disease on Myrtales trees. During recent disease surveys in southern China, sporocarps with fungal pathogen typical characteristics of Cryphonectriaceae were observed on the surfaces of cankers on the stems and branches host jump of Myrtales trees. In this study, a total of 164 Cryphonectriaceae isolates were identified based on comparisons of Myrtaceae DNA sequences of the partial conserved nuclear large subunit (LSU) ribosomal DNA, internal transcribed spacer new taxa (ITS) regions including the 5.8S gene of the ribosomal DNA operon, two regions of the β-tubulin (tub2/tub1) gene, plantation forestry and the translation elongation factor 1-alpha (tef1) gene region, as well as their morphological characteristics. The results showed that eight species reside in four genera of Cryphonectriaceae occurring on the genera Eucalyptus, Melastoma (Melastomataceae), Psidium (Myrtaceae), Syzygium (Myrtaceae), and Terminalia (Combretaceae) in Myrtales. These fungal species include Chrysoporthe deuterocubensis, Celoporthe syzygii, Cel. eucalypti, Cel. guang dongensis, Cel. cerciana, a new genus and two new species, as well as one new species of Aurifilum. These new taxa are hereby described as Parvosmorbus gen. -

Sexual Selection
Sexual Selection Carol E. Lee University of Wisconsin Copyright ©2020 Do not upload without permission Natural Selection Evolutionary Mechanisms Genetic Drift Migration Non-adaptive Mutations Natural Selection Adaptive OUTLINE (1) Sexual Selection (2) Constraints on Natural Selection Pleiotropy Evolutionary Tradeoffs Genetic Drift and Natural Selection ■ There are species with 3 or more sexes (some ciliates have 32)... Too complex to discuss here ■ Restrict my discussion here to 2 sexes Male Female Male Female Male Female HUH???? What about us??? (New World Monkeys) Theory of Sexual Selection ■ The sex bearing a higher cost to reproduction or has higher parental investment will generally be the chooser (has more to lose from bad choice) ■ Whereas the sex bearing the lesser cost of reproduction or parental investment generally competes more heavily for mates (Bateman 1948) Asymmetric Limits on Fitness • The sex that has higher reproductive cost will be the choosers • If you’re allocating a lot of resources toward offspring and are limited in the number of offspring you can have, you won’t mate with just anyone • The sex that is being selected (under sexual selection) will be competitive • Fight with each other, fight for resources, fight for access to or control of the other sex Theory of Sexual Selection ■ In sexual species, it is usually males who invest less in each offspring à it is typical for males to compete for access to females ■ In 90% of mammal species, females provide substantial parental care, while males provide little or -

Supporting Information
Supporting Information Bender et al. 10.1073/pnas.0802779105 Table S1. Species names and calculated numeric values pertaining to Fig. 1 Mitochondrially Nuclear encoded Nuclear encoded Species name encoded methionine, % methionine, % methionine, corrected, % Animals Primates Cebus albifrons 6.76 Cercopithecus aethiops 6.10 Colobus guereza 6.73 Gorilla gorilla 5.46 Homo sapiens 5.49 2.14 2.64 Hylobates lar 5.33 Lemur catta 6.50 Macaca mulatta 5.96 Macaca sylvanus 5.96 Nycticebus coucang 6.07 Pan paniscus 5.46 Pan troglodytes 5.38 Papio hamadryas 5.99 Pongo pygmaeus 5.04 Tarsius bancanus 6.74 Trachypithecus obscurus 6.18 Other mammals Acinonyx jubatus 6.86 Artibeus jamaicensis 6.43 Balaena mysticetus 6.04 Balaenoptera acutorostrata 6.17 Balaenoptera borealis 5.96 Balaenoptera musculus 5.78 Balaenoptera physalus 6.02 Berardius bairdii 6.01 Bos grunniens 7.04 Bos taurus 6.88 2.26 2.70 Canis familiaris 6.54 Capra hircus 6.84 Cavia porcellus 6.41 Ceratotherium simum 6.06 Choloepus didactylus 6.23 Crocidura russula 6.17 Dasypus novemcinctus 7.05 Didelphis virginiana 6.79 Dugong dugon 6.15 Echinops telfairi 6.32 Echinosorex gymnura 6.86 Elephas maximus 6.84 Equus asinus 6.01 Equus caballus 6.07 Erinaceus europaeus 7.34 Eschrichtius robustus 6.15 Eumetopias jubatus 6.77 Felis catus 6.62 Halichoerus grypus 6.46 Hemiechinus auritus 6.83 Herpestes javanicus 6.70 Hippopotamus amphibius 6.50 Hyperoodon ampullatus 6.04 Inia geoffrensis 6.20 Bender et al. www.pnas.org/cgi/content/short/0802779105 1of10 Mitochondrially Nuclear encoded Nuclear encoded Species -

Zambezi After Breakfast, We Follow the Route of the Okavango River Into the Zambezi Where Applicable, 24Hrs Medical Evacuation Insurance Region
SOAN-CZ | Windhoek to Kasane | Scheduled Guided Tour Day 1 | Tuesday 16 ETOSHA NATIONAL PARK 30 Group size Oshakati Ondangwa Departing Windhoek we travel north through extensive cattle farming areas GROUP DAY Katima Mulilo and bushland to the Etosha National Park, famous for its vast amount of Classic: 2 - 16 guests per vehicle CLASSIC TOURING SIZE FREESELL Opuwo Rundu Kasane wildlife and unique landscape. In the late afternoon, once we have reached ETOSHA NATIONAL PARK BWABWATA NATIONAL our camp located on the outside of the National Park, we have the rest of the PARK Departure details Tsumeb day at leisure. Outjo Overnight at Mokuti Etosha Lodge. Language: Bilingual - German and English Otavi Departure Days: Otjiwarongo Day 2 | Wednesday Tour Language: Bilingual DAMARALAND ETOSHA NATIONAL PARK Okahandja The day is devoted purely to the abundant wildlife found in the Etosha Departure days: TUESDAYS National Park, which surrounds a parched salt desert known as the Etosha Gobabis November 17 Pan. The park is home to 4 of the Big Five - elephant, lion, leopard and rhino. 2020 December 1, 15 WINDHOEK Swakopmund Game viewing in the park is primarily focussed around the waterholes, some January 19 of which are spring-fed and some supplied from a borehole, ideal places to February 16 Walvis Bay Rehoboth sit and watch over 114 different game species, or for an avid birder, more than March 2,16,30 340 bird species. An extensive network of roads links the over 30 water holes April 13 SOSSUSVLEI Mariental allowing visitors the opportunity of a comprehensive game viewing safari May 11, 25 throughout the park as each different area will provide various encounters.