A Revised Phylogeny of Nuthatches (Aves, Passeriformes, Sitta) Reveals Insight in Intra- and Interspecific Diversifica- Tion Patterns in the Palearctic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vietnam Southern and Central Specialties 15Th to 29Th February 2020 (15 Days) Trip Report
Vietnam Southern and Central Specialties 15th to 29th February 2020 (15 days) Trip Report Siamese Fireback by Simon Tan Trip report compiled by Tour Leader: Glen Valentine Trip Report – RBL Vietnam – Southern & Central Specialties 2020 2 Tour Summary Orange-breasted Trogon by Simon Tan We kicked off our 2020 Southern and Central Vietnam tour in the city of Hue, teeming with history and cultural significance and some wonderful restaurants, making it the perfect place to spend a few pre-tour days ambling around the city and its surrounds. Beginning our long journey south towards the hill forests surrounding the village of Mang Den, our first scheduled birding stop of the day produced the wanted target species in the form of White-faced Plover (a very distinctive, extremely localized, rare and often- split subspecies of Kentish Plover) along the beach adjacent to Bach Ma National Park. In the same area, we added our first of several Light-vented Bulbuls, a female Stejneger’s Stonechat and flocks of fly-over Grey-capped Greenfinches. Continuing west and then southwards, crossing over the Lo Xo Pass after a quick lunch-stop we had an hour or so to find the scarce and exceedingly range-restricted near-endemic Black-crowned Barwing. This good-looking and charismatic species was only discovered as recently as 1996 and is still only known from a tiny area in south-central Vietnam and southern Laos. We arrived in fine weather and were soon admiring several pairs and family groups of Black-collared Starling, as well as Scarlet Minivet, Streaked Spiderhunter, Black Bulbul, a wonderful perched Necklaced Barbet, a pair of Large Woodshrike and then finally, after a fair amount of searching in the now-degraded road- edge habitat, a glorious pair of Black-crowned Necklaced Barbet by Glen Valentine Rockjumper Birding Tours View more tours to Vietnam Trip Report – RBL Vietnam – Southern & Central Specialties 2020 3 Barwings. -

Poland: May 2015
Tropical Birding Trip Report Poland: May 2015 POLAND The Primeval Forests and Marshes of Eastern Europe May 22 – 31, 2015 Tour Leader: Scott Watson Report and Photos by Scott Watson Like a flying sapphire through the Polish marshes, the Bluethroat was a tour favorite. www.tropicalbirding.com +1-409-515-0514 [email protected] Page1 Tropical Birding Trip Report Poland: May 2015 Introduction Springtime in Eastern Europe is a magical place, with new foliage, wildflowers galore, breeding resident birds, and new arrivals from Africa. Poland in particular is beautiful this time of year, especially where we visited on this tour; the extensive Biebrza Marshes, and some of the last remaining old-growth forest left in Europe, the primeval forests of Bialowieski National Park, on the border with Belarus. Our tour this year was highly successfully, recording 168 species of birds along with 11 species of mammals. This includes all 10 possible Woodpecker species, many of which we found at their nest holes, using the best local knowledge possible. Local knowledge also got us on track with a nesting Boreal (Tengmalm’s) Owl, while a bit of effort yielded the tricky Eurasian Pygmy-Owl and the trickier Hazel Grouse. We also found 11 species of raptors on this tour, and we even timed it to the day that the technicolored European Bee-eaters arrived back to their breeding grounds. A magical evening was spent watching the display of the rare Great Snipe in the setting sun, with Common Snipe “winnowing” all around and the sounds of breeding Common Redshank and Black-tailed Godwits. -

Common Birds of the Prescott Area Nuthatch.Cdr
Birds are listed by their typical habitats. Coniferous Forest Riparian (Creekside) Woodland (Some species may be found in more Sharp-shinned Hawk (w) Anna's Hummingbird (s) than one habitat.) Cooper's Hawk Black-chinned Hummingbird (s) (w) = winter (s) = summer Broad-tailed Hummingbird (s) Rufous Hummingbird (s) Acorn Woodpecker Black Phoebe Pinyon/Juniper/Oak Woodland Red-naped Sapsucker (w) Plumbeous Vireo (s) and Chaparral Hairy Woodpecker Warbling Vireo (s) Gambel's Quail . Western Wood-Pewee (s) House Wren (s) Ash-throated Flycatcher (s) Cordilleran Flycatcher (s) Lucy's Warbler (s) Western Scrub-Jay Hutton's Vireo Yellow Warbler (s) Bridled Titmouse Steller's Jay Summer Tanager (s) Juniper Titmouse . Mountain Chickadee Song Sparrow (w) Bushtit White-breasted Nuthatch Lincoln's Sparrow (w) Bewick's Wren . Pygmy Nuthatch Blue Grosbeak (s) Ruby-crowned Kinglet (w) Brown Creeper Brown-headed Cowbird (s) Blue-gray Gnatcatcher (s) Western Bluebird Bullock's Oriole (s) Crissal Thrasher Hermit Thrush Lesser Goldfinch Phainopepla (s) Yellow-rumped Warbler (w) Lake and Marsh Virginia's Warbler (s) Grace's Warbler (s) Black-throated Gray Warbler (s) Red-faced Warbler (s) Pied-billed Grebe Spotted Towhee Painted Redstart (s) Eared Grebe (w) Canyon Towhee Hepatic Tanager (s) Double-crested Cormorant Rufous-crowned Sparrow Western Tanager (s) Great Blue Heron White-crowned Sparrow (w) Chipping Sparrow Gadwall (w) Black-headed Grosbeak (s) Dark-eyed Junco (w) American Wigeon (w) Scott’s Oriole (s) Pine Siskin Mallard Lake and Marsh (cont.) Varied or Other Habitats Cinnamon Teal (s) Turkey Vulture (s) Common Birds Northern Shoveler (w) Red-tailed Hawk Northern Pintail (w) Rock Pigeon of the Green-winged Teal (w) Eurasian Collared-Dove Prescott Canvasback (w) Mourning Dove Ring-necked Duck (w) Greater Roadrunner Area Lesser Scaup (w) Ladder-backed Woodpecker Bufflehead (w) Northern Flicker Common Merganser (w) Cassin's Kingbird (s) Ruddy Duck (w) Common Raven American Coot Violet-green Swallow (s) . -

Wallcreeper and the Camargue
Wallcreeper and The Camargue Naturetrek Tour Report 19 – 23 November 2018 Firecrest Western Swamphen Stripeless Tree Frog Spoonbill Report compiled by Matt Collis Images courtesy of Neil McMahon Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Wallcreepers and The Camargue Tour participants: Matt Collis & Neil McMahon (leaders) with 16 Naturetrek clients Summary A short birding trip to the very special Camargue district of southern France provides an excellent opportunity to experience good views of typical waterbirds of the west Mediterranean. Utilising a family-run hotel on the outskirts of the ancient city of Arles as our base, the itinerary provided an easy opportunity to look for flamingoes, herons and wading birds using the shallow waters and reed-fringed lagoons as a breeding area or a suitable stop-over for migrants. The mountainous areas attract different and more localized species and we located special birds such as Wallcreeper, Rock Sparrow and Booted Eagle, together with a selection of other stunning species that call the Camargue home. Day 1 Monday 19th November Arriving in the early evening, leaders Neil and Matt met 15 of the 16 clients at Marseille airport, before collecting the minibuses and beginning the journey to the hotel. French protests and road blocks meant the route was more cross country but after just over an hour or so we arrived at our destination, Hotel des Granges. We were greeted by Bruno and Marie-Jo, our wonderful hosts and owners of this classically French Hotel, and a lone Black Redstart, the first bird for our trip, sat roosting over the entrance porch. -

PPCO Twist System
A Brown-headed Nuthatch brings food to the nest. Color-coded leg bands enable the authors and their colleagues to discern the curious and potentially complex relationships among the various individuals at Tall Timbers Research Station on the Florida–Georgia line. Photo by © Tara Tanaka. photos by Tara Tanaka Tallahassee, Florida [email protected] 46 BIRDING • FEBRUARY 2016 arblers are gorgeous, jays boisterous, and sparrows elusive, Wbut words like “cute” and “adorable” come to mind when the conversation shifts to Brown-headed Nuthatches. The word “cute” doesn’t appear in the scientifc literature regularly, but science may help to explain its frequent association with nuthatch- es. The large heads and small bodies nuthatches possess have propor- tions similar to those found on young children. Psychologists have found that these proportions conjure up “innate attractive” respons- es among adult humans even when the proportions fall on strange objects (Little 2012). This range-restricted nuthatch associated with southeastern pine- woods has undergone steep declines in recent decades and is listed as a species of special concern in most states in which it breeds (Cox and Widener 2008). The population restricted to Grand Bahama Island appears to be critically endangered (Hayes et al. 2004). Add to these concerns some intriguing biology that includes helpers at the nest, communal winter roosts, seed caching, social grooming, and the use of tools, and you have some scintillating science all bound up in one “cute” and “adorable” package. James -

Melagiris (Tamil Nadu)
MELAGIRIS (TAMIL NADU) PROPOSAL FOR IMPORTANT BIRD AREA (IBA) State : Tamil Nadu, India District : Krishnagiri, Dharmapuri Coordinates : 12°18©54"N 77°41©42"E Ownership : State Area : 98926.175 ha Altitude : 300-1395 m Rainfall : 620-1000 mm Temperature : 10°C - 35°C Biographic Zone : Deccan Peninsula Habitats : Tropical Dry Deciduous, Riverine Vegetation, Tropical Dry Evergreen Proposed Criteria A1 (Globally Threatened Species) A2 (Endemic Bird Area 123 - Western Ghats, Secondary Area s072 - Southern Deccan Plateau) A3 (Biome-10 - Indian Peninsula Tropical Moist Forest, Biome-11 - Indo-Malayan Tropical Dry Zone) GENERAL DESCRIPTION The Melagiris are a group of hills lying nestled between the Cauvery and Chinnar rivers, to the south-east of Hosur taluk in Tamil Nadu, India. The Melagiris form part of an almost unbroken stretch of forests connecting Bannerghatta National Park (which forms its north-western boundary) to the forests of Cauvery Wildlife Sanctuary - Karnataka (which forms its southern boundary, separated by the river Cauvery), and further to Biligirirangan hills and Sathyamangalam forests. The northern and western parts are comparatively plain and is part of the Mysore plateau. The average elevation in this region is 500-1000 m. Ground sinks to 300m in the valley of the Cauvery and the highest point is the peak of Guthereyan at 1395.11 m. Red sandy loam is the most common soil type found in this region. Small deposits of alluvium are found along Cauvery and Chinnar rivers and Kaoline is found in some areas near Jowlagiri. The temperature ranges from 10°C ± 35°C. South-west monsoon is fairly active mostly in the northern areas, but north-east monsoon is distinctly more effective in the region. -

December 2013
December, 2013 TALL TALES It is once again time for everyone to practice a tale about the wild experiences everyone has about our fun pastime birding. Our annual year's end program of TALL TALES will end our club's meetings for 2013. So, be prepared to share your wild birding experience from the Christmas Count or one of your field trips or your backyard feeder, or your trip to Timbuktu. Make it exciting for everyone to help relive your memory. Bring friends and let them share in the excitement and the holiday refreshments. Our meetings are held September through June on the third Monday of each month. They begin at 7 p.m. at the Will Rogers Garden Center, I-44 & NW 36th Street. Visitors are always welcome. REFRESHMENTS Everyone, please bring something savory or sweet to share at our annual Christmas meeting. Drinks will be provided. WELCOME New Member: Calvin Rees, Oklahoma City DUES NOTICE It's time once again to renew your annual membership for 2014. OCAS dues are $15 per household and may be paid at the monthly meetings, online at our website http://www.okc-audubon.org or mailed to Nancy Vicars, Treasurer, 24 SE 57th St., OKC 73129 . MEETING CHANGE Mark your calendar now!!! The January meeting will be held on the 4th Monday, January 27, 2014 due to the Garden Center closure for Martin Luther King Jr. Day. three families are in the superfamily Meliphagoidea in the President’s Perspective basal songbird Australasian grade (BSAG supergroup in By Bill Diffin this series) which consists of all ten of the terminal clades below Passerida and Corvoidea in the ToL Hi, Members. -
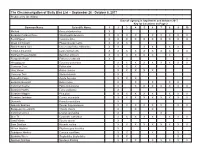
The Circumnavigation of Sicily Bird List -- September 26
The Circumnavigation of Sicily Bird List -- September 26 - October 8, 2017 Produced by Jim Wilson Date of sighting in September and October 2017 Key for Locations on Page 2 Common Name Scientific Name 1 2 3 4 5 6 7 8 9 10 Mallard Anas platyrhynchos X Eurasian Collared Dove Streptopelia decaocto X X X X X X X X X Feral Pigeon Columba livia X X X X X X X X X X Great Cormorant Phalacrocorax carbo X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X Yellow-legged Gull Larus michahellis X X X X X X X X X X Northern House Martin Delichon urbicum X X X X X X X European Robin Erithacus rubecula X X Woodpigeon Columba palumbus X X X X X X X X X Common Coot Fulica atra X X X Grey Heron Ardea cinerea X X X X X Common Tern Sterna hirundo X Bonnelli's Eagle Aquila fasciata X X X Eurasian Buzzard Buteo buteo X X X X X Common Kestrel Falco tinnunculus X X X X X X X X Eurasian Hobby Falco subbuteo X Eurasian Magpie Pica pica X X X X X X X X Eurasian Jackdaw Corvus monedula X X X X X X X X X Dunnock Prunella modularis X Spanish Sparrow Passer hispaniolensis X X X X X X X X European Greenfinch Chloris chloris X X X Common Linnet Linaria cannabina X X Blue Tit Cyanistes caeruleus X X X X X X X X Sand Martin Riparia riparia X Barn Swallow Hirundo rustica X X X X X X X X X Willow Warbler Phylloscopus trochilus X Subalpine Warbler Curruca cantillans X X X X Eurasian Wren Troglodytes troglodytes X X X Spotless Starling Spotless Starling X X X X X X X X Common Name Scientific Name 1 2 3 4 5 6 7 8 9 10 Sardinian Warbler Curruca melanocephala X X X X -
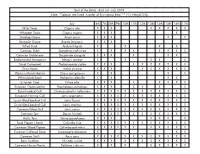
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Wildlife Ecology Provincial Resources
MANITOBA ENVIROTHON WILDLIFE ECOLOGY PROVINCIAL RESOURCES !1 ACKNOWLEDGEMENTS We would like to thank: Olwyn Friesen (PhD Ecology) for compiling, writing, and editing this document. Subject Experts and Editors: Barbara Fuller (Project Editor, Chair of Test Writing and Education Committee) Lindsey Andronak (Soils, Research Technician, Agriculture and Agri-Food Canada) Jennifer Corvino (Wildlife Ecology, Senior Park Interpreter, Spruce Woods Provincial Park) Cary Hamel (Plant Ecology, Director of Conservation, Nature Conservancy Canada) Lee Hrenchuk (Aquatic Ecology, Biologist, IISD Experimental Lakes Area) Justin Reid (Integrated Watershed Management, Manager, La Salle Redboine Conservation District) Jacqueline Monteith (Climate Change in the North, Science Consultant, Frontier School Division) SPONSORS !2 Introduction to wildlife ...................................................................................7 Ecology ....................................................................................................................7 Habitat ...................................................................................................................................8 Carrying capacity.................................................................................................................... 9 Population dynamics ..............................................................................................................10 Basic groups of wildlife ................................................................................11 -

Status and Phylogenetic Analyses of Endemic Birds of the Himalayan Region
Pakistan J. Zool., vol. 47(2), pp. 417-426, 2015. Status and Phylogenetic Analyses of Endemic Birds of the Himalayan Region M.L. Thakur* and Vineet Negi Himachal Pradesh State Biodiversity Board; Department of Environment, Science & Technology, Shimla-171 002 (HP), India Abstract.- Status and distribution of 35 species of birds endemic to Himalayas has been analysed during the present effort. Of these, relatively very high percentage i.e. 46% (16 species) is placed under different threat categories. Population of 24 species (74%) is decreasing. A very high percentage of these Himalayan endemics (88%) are dependent on forests. Population size of most of these bird species (22 species) is not known. Population size of some bird species is very small. Distribution area size of some of the species is also very small. Three species of endemic birds viz., Callacanthis burtoni, Pyrrhula aurantiaca and Pnoepyga immaculata appear to have followed some independent evolutionary lineage and also remained comparatively stable over the period of time. Three different evolutionary clades of the endemic bird species have been observed on the basis of phylogenetic tree analyses. Analyses of length of branches of the phylogenetic tree showed that the three latest entries in endemic bird fauna of Himalayan region i.e. Catreus wallichii, Lophophorus sclateri and Tragopan blythii have been categorised as vulnerable and therefore need the highest level of protection. Key Words: Endemic birds, Himalayan region, conservation status, phylogeny. INTRODUCTION broadleaved forests in the mid hills, mixed conifer and coniferous forests in the higher hills, and alpine meadows above the treeline mainly due to abrupt The Himalayas, one of the hotspots of rise of the Himalayan mountains from less than 500 biodiversity, include all of the world's mountain meters to more than 8,000 meters (Conservation peaks higher than 8,000 meters, are stretched in an International, 2012). -

An Update of Wallacels Zoogeographic Regions of the World
REPORTS To examine the temporal profile of ChC produc- specification of a distinct, and probably the last, 3. G. A. Ascoli et al., Nat. Rev. Neurosci. 9, 557 (2008). tion and their correlation to laminar deployment, cohort in this lineage—the ChCs. 4. J. Szentágothai, M. A. Arbib, Neurosci. Res. Program Bull. 12, 305 (1974). we injected a single pulse of BrdU into pregnant A recent study demonstrated that progeni- CreER 5. P. Somogyi, Brain Res. 136, 345 (1977). Nkx2.1 ;Ai9 females at successive days be- tors below the ventral wall of the lateral ventricle 6. L. Sussel, O. Marin, S. Kimura, J. L. Rubenstein, tween E15 and P1 to label mitotic progenitors, (i.e., VGZ) of human infants give rise to a medial Development 126, 3359 (1999). each paired with a pulse of tamoxifen at E17 to migratory stream destined to the ventral mPFC 7. S. J. Butt et al., Neuron 59, 722 (2008). + 18 8. H. Taniguchi et al., Neuron 71, 995 (2011). label NKX2.1 cells (Fig. 3A). We first quanti- ( ). Despite species differences in the develop- 9. L. Madisen et al., Nat. Neurosci. 13, 133 (2010). fied the fraction of L2 ChCs (identified by mor- mental timing of corticogenesis, this study and 10. J. Szabadics et al., Science 311, 233 (2006). + phology) in mPFC that were also BrdU+. Although our findings raise the possibility that the NKX2.1 11. A. Woodruff, Q. Xu, S. A. Anderson, R. Yuste, Front. there was ChC production by E15, consistent progenitors in VGZ and their extended neurogenesis Neural Circuits 3, 15 (2009).