Information for the Patient ESMOLOL HYDROCHLORIDE 2500 Mg
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Use of Intravenous Esmolol to Predict Efficacy of Oral Beta-Adrenergic Blocker Therapy in Patients with Neurocardiogenic Syncope
402 JACC Vol. 19, No. 2 February 1992:402-8 REPORTS ON THERAPY Use of Intravenous Esmolol to Predict Efficacy of Oral Beta Adrenergic Blocker Therapy in Patients With Neurocardiogenic Syncope JASBIR S. SRA, MD, VISHNUBHAKTA S. MURTHY, MD, PHD, MOHAMMAD R. JAZAYERI, MD, FACC, YUE-HUA SHEN, MD, PAUL J. TROUP, MD, FACC, BOAZ AVITALL, MD, PHD, MASOOD AKHTAR, MD, FACC Milwaukee, Wisconsin The usefulness of esmolol in predicting the efficacy of treatment Irrespective of their response to esmolol infusion, all patients with an oral beta-adrenergic blocking agent was evaluated in 27 had a follow-up tilt test with oral metoprolol after an interval of consecutive patients with neurocardiogenic syncope. Seventeen ~5 half-lives of the drug. All 16 patients (100%) with a negative patients had a positive head-up tilt test response at baseline and 10 tilt test response during esmolol infusion had a negative tilt test patients required intravenous isoproterenol for provocation of response with oral metoprolol. Of the 11 patients with a positive hypotension. All patients were then given a continuous esmolol tilt test response during esmolol infusion, 10 (90%) continued to infusion (500 JLg/kg per min loading dose for 3 min followed by have a positive response with oral metoprolol. 300 Jlg/kg per min maintenance dose) and rechallenged with a It is concluded that in the electrophysiology laboratory, es head-up tilt test at baseline or with isoproterenol. molol can accurately predict the outcome of a head-up tilt Of the 17 patients with a positive baseline tilt test response, 11 response to oral metoprolol. -
Esmolol Hydrochloride 10 Mg/Ml Solution for Injection Esmolol Hydrochloride
Package leaflet: Information for the patient Esmolol hydrochloride 10 mg/ml solution for injection Esmolol hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. – Keep this leaflet. You may need to read it again. – If you have any further questions, ask your doctor or nurse. – This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. – If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Esmolol hydrochloride 10 mg/ml solution for injection is and what it is used for 2. What you need to know before you use Esmolol hydrochloride 10 mg/ml solution for injection 3. How to use Esmolol hydrochloride 10 mg/ml solution for injection 4. Possible side effects 5. How to store Esmolol hydrochloride 10 mg/ml solution for injection 6. Contents of the pack and other information 1. What Esmolol hydrochloride 10 mg/ml chlor promazine, used to treat mental dis - solution for injection is and what it is orders) used for – Clozapine which is used to treat mental Esmolol hydrochloride 10 mg/ml belongs to the disorders group of beta-blockers. These medicines slow – Epinephrine, which is used to treat allergic down the heartbeat and reduce blood pressure. reactions – Medicines used to treat asthma Esmolol hydrochloride 10 mg/ml is used for – Medicines used to treat colds or a blocked short-term treatment if your heart beats too nose, called nasal “decongestants” fast. -
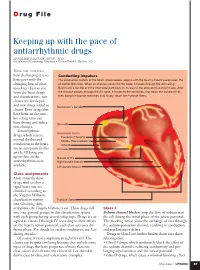
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Comparative Study of IV Esmolol, IV Diltiazem, and IV Lignocaine Hydrochloride in Attenuating Pressure Response to Laryngoscopy and Intubation
Jemds.com Original Research Article Comparative Study of IV Esmolol, IV Diltiazem, and IV Lignocaine Hydrochloride in Attenuating Pressure Response to Laryngoscopy and Intubation Sathya Narayanan Karunanithi1, Geeta Bhandari2, Kedar Singh Shahi3, Aditya Kumar Chauhan4, Pooja Gautam5 1Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 2 Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 3Department of Surgery, Government Medical College, Haldwani, Uttarakhand, India. 4Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. 5Department of Anaesthesiology, Critical Care, Pain and Palliative Medicine Government Medical College, Haldwani, Uttarakhand, India. ABSTRACT BACKGROUND Not many studies have compared more than two drugs in attenuating pressor Corresponding Author: responses to laryngoscopy and intubation. This study compares four groups of Dr. Geeta Bhandari, considerable size. The present study compared intravenous esmolol, diltiazem, and Professor and HOD, Department of Anaesthesiology, lignocaine, for their efficacy to abate pressure response to laryngoscopy and Critical Care, Pain and Palliative Medicine, intubation. Government Medical College, Haldwani-263139, Uttarakhand, India. METHODS E-mail: [email protected] This is a prospective, randomized, double-blinded, controlled clinical study conducted among 220 patients of ASA grade I/II (age 18–60 years), undergoing DOI: 10.14260/jemds/2020/447 elective surgical procedure requiring general anaesthesia with endotracheal intubation over a period of 15 months at a tertiary hospital setup. Study subjects How to Cite This Article: were categorised as Groups D, E, L, and N that received diltiazem (0.2 mg/Kg IV), Karunanithi SN, Bhandari G, Shahi KS, et esmolol (2 mg/Kg IV), lignocaine (1.5 mg/Kg IV), and normal saline, respectively; al. -

Atrial Fibrillation in the Surgical Patient
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care practices based upon the available medical literature and clinical expertise at the time of development. They should not be considered to be accepted protocol or policy, nor are intended to replace clinical judgment or dictate care of individual patients. NEW ONSET ATRIAL FIBRILLATION IN THE SURGICAL PATIENT SUMMARY Atrial fibrillation is a common postoperative arrhythmia and can represent a major source of morbidity and mortality. Treatment of atrial fibrillation is directed at three main objectives: controlling the ventricular response, preventing thromboembolism, and maintaining sinus rhythm. Therapeutic decisions also hinge on patients’ hemodynamic stability. In patients who are hemodynamically unstable, direct current cardioversion is the first line therapy and pharmacotherapy should be used as adjunctive treatment. In patients who are hemodynamically stable, pharmacologic treatment including class II (beta-blockers), class III (amiodarone), or class IV (nondihydropyridine calcium channel blockers) agents are viable options. RECOMMENDATIONS Level 1 Beta-blockade (esmolol or metoprolol), nondihydropyridine calcium channel blockers (diltiazem or verapamil), and amiodarone are pharmacologic options to manage new onset atrial fibrillation (see table for dosing). Beta-blockers are the first line therapy for postoperative atrial fibrillation to achieve rapid ventricular rate control and conversion to sinus rhythm. Diltiazem is second line rate control agent when beta-blocker therapy has failed. Both therapies should be avoided in hypotensive patients. Amiodarone can provide both rate and rhythm control and is an alternative therapy to beta-blockade for postoperative atrial fibrillation especially when the patient is hemodynamically unstable or has a known ejection fraction of < 40%. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Comparison of the Β-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacoc
1521-0103/359/1/73–81$25.00 http://dx.doi.org/10.1124/jpet.116.232884 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 359:73–81, October 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Comparison of the b-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions Shahrooz Nasrollahi-Shirazi, Sonja Sucic, Qiong Yang, Michael Freissmuth, and Christian Nanoff Institute of Pharmacology, Center of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria Received February 15, 2016; accepted July 18, 2016 Downloaded from ABSTRACT Blockage of b1-adrenergic receptors is one of the most effective was very low. Both landiolol and esmolol caused a very modest treatments in cardiovascular medicine. Esmolol was introduced rise in cAMP levels but a robust increase in the phosphorylation some three decades ago as a short-acting b1-selective antag- of extracellular signal regulated kinases 1 and 2, indicating that the onist. Landiolol is a more recent addition. Here we compared the two drugs exerted partial agonist activity with a signaling bias. If two compounds for their selectivity for b1-adrenergic receptors cells were incubated for $24 hours in the presence of $1 mM jpet.aspetjournals.org over b2-adrenergic receptors, partial agonistic activity, signaling esmolol, the levels of b1-adrenergic—but not of b2-adrenergic— bias, and pharmacochaperoning action by using human embry- receptors increased. This effect was contingent on export of onic kidney (HEK)293 cell lines, which heterologously express the b1-receptor from endoplasmic reticulum and was not seen each human receptor subtype. -

Drugs That Affect the Cardiovascular System
PharmacologyPharmacologyPharmacology DrugsDrugs thatthat AffectAffect thethe CardiovascularCardiovascular SystemSystem TopicsTopicsTopics •• Electrophysiology Electrophysiology •• Vaughn-Williams Vaughn-Williams classificationclassification •• Antihypertensives Antihypertensives •• Hemostatic Hemostatic agentsagents CardiacCardiacCardiac FunctionFunctionFunction •• Dependent Dependent uponupon –– Adequate Adequate amountsamounts ofof ATPATP –– Adequate Adequate amountsamounts ofof CaCa++++ –– Coordinated Coordinated electricalelectrical stimulusstimulus AdequateAdequateAdequate AmountsAmountsAmounts ofofof ATPATPATP •• Needed Needed to:to: –– Maintain Maintain electrochemicalelectrochemical gradientsgradients –– Propagate Propagate actionaction potentialspotentials –– Power Power musclemuscle contractioncontraction AdequateAdequateAdequate AmountsAmountsAmounts ofofof CalciumCalciumCalcium •• Calcium Calcium isis ‘glue’‘glue’ that that linkslinks electricalelectrical andand mechanicalmechanical events.events. CoordinatedCoordinatedCoordinated ElectricalElectricalElectrical StimulationStimulationStimulation •• Heart Heart capablecapable ofof automaticityautomaticity •• Two Two typestypes ofof myocardialmyocardial tissuetissue –– Contractile Contractile –– Conductive Conductive •• Impulses Impulses traveltravel throughthrough ‘action‘action potentialpotential superhighway’.superhighway’. A.P.A.P.A.P. SuperHighwaySuperHighwaySuperHighway •• Sinoatrial Sinoatrial node node •• Atrioventricular Atrioventricular nodenode •• Bundle Bundle ofof -

SUPPLEMENTARY MATERIAL 1: Search Strategy
SUPPLEMENTARY MATERIAL 1: Search Strategy Medline search strategy 1. exp basal ganglia hemorrhage/ or intracranial hemorrhages/ or cerebral hemorrhage/ or intracranial hemorrhage, hypertensive/ or cerebrovascular disorders/ 2. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or pon$ or lentiform$ or brainstem or cortic$ or cortex$ or subcortic$ or subcortex$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw 3. ((hemorrhag$ or haemorrhag$) adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw 4. (ICH or ICHs or PICH or PICHs).tw 5. 1 or 2 or 3 or 4 6. exp blood pressure/ 7. exp hypertension/ 8. (blood pressure or bloodpressure).tw 9. ((bp or blood pressure) adj5 (lowering or reduc$)).tw 10. ((strict$ or target$ or tight$ or intens$ or below) adj3 (blood pressure or systolic or diastolic or bp or level$)).tw 11. (hypertension or hypertensive).tw 12. ((manage$ or monitor$) adj3 (hypertension or blood pressure)).tw 13. ((intense or intensive or aggressive or accelerated or profound or radical or severe) adj5 ((bp or blood pressure) adj5 (lowering or reduc$ or decreas$ or decrement or dimin$ or declin$))).tw 14. ((standard or normal or ordinary or guideline or guide line or guideline recommend$ or recommend$ or convention$ or usual or established) adj5 ((bp or blood pressure) adj5 (lowering or reduc$ or decreas$ or decrement or dimin$ or declin$))).tw 15. (antihypertensive adj2 (agent$ or drug$ or medicat$)).tw 1 16. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. -

Get in Rhythm with the Safe and Effective Use of Antiarrythmic Drugs
Get in Rhythm with the Safe and Effective Use of Antiarrythmic Drugs Karen J. McConnell, Pharm.D., FCCP, BCPS- AQ Cardiology, ASH-CHC Clinical Director and Cardiology Subject Matter Expert, Cardinal Health Clinical Associate Professor, University of Colorado Skaggs School of Pharmacy and Shannon W. Finks, Pharm.D., FCCP, BCPS- AQ Cardiology, ASH-CHC Professor of Clinical Pharmacy, University of Tennessee College of Pharmacy Clinical Specialist Cardiology, Veterans Affairs Medical Center Memphis Disclosure The program chair and presenters for this continuing education activity have reported no relevant financial relationships. Objectives . Design patient-specific treatment and monitoring plans for antiarrhythmic drugs (AADs) to treat atrial fibrillation (AF) . Differentiate among appropriate monitoring strategies for various agents used in ventricular arrhythmia suppression . Avoid potential adverse drug events with AADs by identifying important drug interactions . Ensure safe and effective dosing of AADs based upon specific patient factors Rhythm Rule #1 . Pharmacists play a vital role in the appropriate use of AAD dosing, adverse effects, interactions, and monitoring. Treatment and Monitoring of Atrial Fibrillation Atrial Fibrillation • Most common type of serious arrhythmia • In U.S., affects 2-5 million patients • Frequently seen with comorbidities • AF complicates management of comorbidity • Comorbidity complicates management of AF • Associated with stroke, heart failure, death • Most common arrhythmia requiring hospitalization Case #1: Mary Rhythm 60 y/o AA woman with a PMH including . Laboratory data: HFrEF (EF 35%), atrial fibrillation(AF), CKD, 140 110 18 HTN 105 . Inpatient Medications: 4.7 22 1.6 apixaban 5 mg twice daily lisinopril 20 mg daily BP 115/78 mm Hg HT 67 in metoprolol succinate 50 mg/day HR 70 bpm WT 75 kg furosemide 40 mg twice daily spironolactone 25 mg/day . -

A Comparison of Intravenous Esmolol Versus Intravenous Diltiazem in Atrial Fibrillation with Rapid Ventricular Response-A Study from an Academic Center
Cardiol Cardiovasc Med 2020; 4 (4): 450-459 DOI: 10.26502/fccm.92920143 Research Article A Comparison of Intravenous Esmolol versus Intravenous Diltiazem in Atrial Fibrillation with Rapid Ventricular Response-A Study from An Academic Center Manjari R Regmi1*, Mohammad Al-Akchar2, Bishal Bhandari1, Abdisamad M Ibrahim1, Priyanka Parajuli1, Odalys Estefania Lara Garcia1, Basma Al-Bast1, Nitin Tandan1, Ruby Maini1, Warren Alexander Skoza1, Marissa Kircher1, Abigail Levy1, Joseph Pflederer1, Brendan Finnell1, Albert Botchway1, Vivek Prakash1, Abhishek Kulkarni2, Momin Siddique2, Mohamed Labedi2 1Department of Internal Medicine, Southern Illinois University School of Medicine, Springfield, IL, USA 2Division of Cardiology, Southern Illinois University School of Medicine, Springfield, IL, USA *Corresponding Authors: Manjari Rani Regmi, Southern Illinois University School of Medicine, 701 North First Street, PO Box 19636, Springfield, IL 62794-9636, USA, Tel: 217-545-0193; F a x : 279-201-6023; E-m a i l : [email protected] Received: 03 August 2020; Accepted: 15 August 2020; Published: 26 August 2020 Citation: Manjari R Regmi, Mohammad Al-Akchar, Bishal Bhandari, Abdisamad M Ibrahim, Priyanka Parajuli, Odalys Estefania Lara Garcia, Basma Al-Bast, Nitin Tandan, Ruby Maini, Warren Alexander Skoza, Marissa Kircher, Abigail Levy, Joseph Pflederer, Brendan Finnell, Albert Botchway, Vivek Prakash, Abhishek Kulkarni, Momin Siddique, Mohamed Labedi. A Comparison of Intravenous Esmolol versus Intravenous Diltiazem in Atrial Fibrillation with Rapid Ventricular Response-A Study from An Academic Center. Cardiology and Cardiovascular Medicine 4 (2020): 450-459. Abstract Introduction: This study’s objective was to compare Methods: The measured parameters were the number of the differences in the effects of intravenous esmolol and hospital re-admissions, length of hospital stay, time diltiazem on patients having atrial fibrillation with taken for rate control, and side effects like hypotension RVR. -

The Effect of Intraoperative Lidocaine Versus Esmolol Infusion On
Bajracharya et al. BMC Anesthesiology (2019) 19:198 https://doi.org/10.1186/s12871-019-0874-8 RESEARCH ARTICLE Open Access The effect of intraoperative lidocaine versus esmolol infusion on postoperative analgesia in laparoscopic cholecystectomy: a randomized clinical trial Joshan Lal Bajracharya1, Asish Subedi2* , Krishna Pokharel3 and Balkrishna Bhattarai4 Abstract Background: As a part of multimodal analgesia for laparoscopic cholecystectomy, both intraoperative lidocaine and esmolol facilitate postoperative analgesia. Our objective was to compare these two emerging strategies that challenge the use of intraoperative opioids. We aimed to assess if intraoperative esmolol infusion is not inferior to lidocaine infusion for opioid consumption after laparoscopic cholecystectomy. Methods: In this prospective, randomized, double-blind, non-inferiority clinical trial, 90 female patients scheduled for elective laparoscopic cholecystectomy received either intravenous (IV) lidocaine bolus 1.5 mg/kg at induction followed by an infusion (1.5 mg/ kg/h) or IV bolus of esmolol 0.5 mg/kg at induction followed by an infusion (5– 15 μg/kg/min) till the end of surgery. Remaining aspect of anesthesia followed a standard protocol apart from no intraoperative opioid supplementation. Postoperatively, patients received either morphine or tramadol IV to maintain visual analogue scale (VAS) scores ≤3. The primary outcome was opioid consumption (in morphine equivalents) during the first 24 postoperative hours. Pain and sedation scores, time to first perception of pain and void, and occurrence of nausea/vomiting were secondary outcomes measured up to 24 h postoperatively. Results: Two patients in each group were excluded from the analysis. The postoperative median (IQR) morphine equivalent consumption in patients receiving esmolol was 1 (0–1.5) mg compared to 1.5 (1–2) mg in lidocaine group (p = 0.27).