Participant List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Katakami et al. Cardiovasc Diabetol (2020) 19:110 https://doi.org/10.1186/s12933-020-01079-4 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Tofoglifozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3 and Iichiro Shimomura1 on behalf of the UTOPIA study investigators Abstract Background: This study aimed to investigate the preventive efects of tofoglifozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT). Methods: This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofoglifozin treatment group (n 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n 171). Primary outcomes were changes= in mean and maximum common carotid IMT measured by echography during= a 104-week treatment period. Results: In a mixed-efects model for repeated measures, the mean IMT of the common carotid artery (mean- IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), signifcantly declined in both the tofoglifozin ( 0.132 mm, SE 0.007; 0.163 mm, SE 0.013; 0.170 mm, SE 0.020, respectively) and the control group ( 0.140 mm,− SE 0.006; 0.190 mm,− SE 0.012; 0.190 mm,− SE 0.020, respectively). -

Downloads but Many Other Countries — Such As the RCMP Said the Policy Revisions /JJ Release on April30.Pdf)
CMAJ News For the record Published at www.cmaj.ca between Apr. The new warning letter regards in persistent or significant disability or 20 and May 18 breaches uncovered during a July– incapacity, be life-threatening or result August 2009 FDA inspection of an in death. Apotex receives second FDA Apotex facility located at 150 Signet The report indicates that there has Drive in Toronto, during which “sev- been a steady increase of adverse reac- warning eral violations that are identical” to tion reports since 2001, when roughly those found during the inspection of the 11 000 incidents were reported (www.hc he United States Food and Drug Etobicoke facility were discovered. -sc.gc.ca/dhp-mps/alt_formats/pdf/med Administration (FDA) has “These identical CGMP violations eff/bulletin/carn-bcei_v20n2-eng.pdf). T issued a second warning letter demonstrated a lack of adequate Roughly 70% (18 301) of 2009 inci- in a year to Toronto, Ontario-based process controls and raised serious dents involved pharamaceuticals, while generic drug manufacturer Apotex Inc. questions regarding your corporation’s 23% (5998) involved biotechnology for lapses in good manufacturing prac- quality and production systems,” the products. There were 833 adverse reac- tices. warning letter states. tion reports involving biologics, 516 The violations cause Apotex drug The violations included contamina- involving natural health products, 379 products to be considered “adulterated” tion of a diabetes drug with an “active involving radiopharmaceuticals and 34 within US regulations -

Amgen 2008 Annual Report and Financial Summary Pioneering Science Delivers Vital Medicines
Amgen 2008 Annual Report and Financial Summary Pioneering science delivers vital medicines About Amgen Products Amgen discovers, develops, manufactures Aranesp® (darbepoetin alfa) and delivers innovative human therapeutics. ® A biotechnology pioneer since 1980, Amgen Enbrel (etanercept) was one of the fi rst companies to realize ® the new science’s promise by bringing EPOGEN (Epoetin alfa) safe and effective medicines from the lab Neulasta® (pegfi lgrastim) to the manufacturing plant to patients. NEUPOGEN® (Filgrastim) Amgen therapeutics have changed the practice of medicine, helping millions of Nplate® (romiplostim) people around the world in the fi ght against ® cancer, kidney disease, rheumatoid Sensipar (cinacalcet) arthritis and other serious illnesses, and so Vectibix® (panitumumab) far, more than 15 million patients worldwide have been treated with Amgen products. With a broad and deep pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people’s lives. 0405 06 07 08 0405 06 07 08 0405 06 07 08 0405 06 07 08 Total revenues “Adjusted” earnings Cash fl ow from “Adjusted” research and ($ in millions) per share (EPS)* operations development (R&D) expenses* (Diluted) ($ in millions) ($ in millions) 2008 $15,003 2008 $4.55 2008 $5,988 2008 $2,910 2007 14,771 2007 4.29 2007 5,401 2007 3,064 2006 14,268 2006 3.90 2006 5,389 2006 3,191 2005 12,430 2005 3.20 2005 4,911 2005 2,302 2004 10,550 2004 2.40 2004 3,697 2004 1,996 * “ Adjusted” EPS and “adjusted” R&D expenses are non-GAAP fi nancial measures. See page 8 for reconciliations of these non-GAAP fi nancial measures to U.S. -

Large Cap Value Fund Q3 Portfolio Holdings
Putnam Equity Income Fund The fund's portfolio 8/31/20 (Unaudited) COMMON STOCKS (95.5%)(a) Shares Value Aerospace and defense (3.0%) Northrop Grumman Corp. 763,179 $261,472,757 Raytheon Technologies Corp. 1,931,815 117,840,715 379,313,472 Airlines (1.0%) Southwest Airlines Co. 3,596,011 135,138,093 135,138,093 Auto components (0.8%) Delphi Automotive PLC 1,165,950 100,411,614 100,411,614 Automobiles (0.7%) General Motors Co. 2,932,830 86,899,753 86,899,753 Banks (9.9%) Bank of America Corp. 13,888,550 357,491,277 Citigroup, Inc. 6,615,251 338,171,631 JPMorgan Chase & Co. 3,413,635 342,012,091 KeyCorp 3,945,705 48,611,086 PNC Financial Services Group, Inc. (The) 1,279,414 142,270,837 1,228,556,922 Beverages (2.5%) Keurig Dr Pepper, Inc.(S) 3,097,349 92,393,921 Molson Coors Beverage Co. Class B(S) 1,663,844 62,627,088 PepsiCo, Inc. 1,123,779 157,396,487 312,417,496 Biotechnology (4.5%) AbbVie, Inc. 2,005,931 192,108,012 Amgen, Inc. 804,202 203,720,451 Regeneron Pharmaceuticals, Inc.(NON) 262,080 162,471,254 558,299,717 Building products (2.3%) Fortune Brands Home & Security, Inc. 1,681,051 141,342,768 Johnson Controls International PLC 3,432,811 139,818,392 281,161,160 Capital markets (3.5%) Apollo Global Management, Inc. 2,289,153 107,292,601 Charles Schwab Corp. (The) 2,516,793 89,421,655 Goldman Sachs Group, Inc. -

Sigma Spectrum Operator's Manual
spectrum Operator’s Manual 35700BAX & 35700ABB Generation 2 Operating System Pump Operating Software v6.05 For Use with MDL Editor v6.2.4 SIGMA, LLC 711 Park Avenue Medina, New York 14103 v 800 356 3454 f 585 798 3909 www.sigmapumps.com Manual 41018- 6.05/6.2.4 Revision D II Pump Operating Software v6.05 For Use With MDL Editor v6.2.4 ©2011 Copyright SIGMA, LLC 711 Park Avenue Medina, New York 14103 v 800 356 3454 f 585 798 3909 www.sigmapumps.com III Manual 41018- 6.05/6.2.4 Revision D IV Pump Operating Software v6.05 For Use With MDL Editor v6.2.4 TABLE OF CONTENTS Introduction and Safety . 1 Intended Device Use . 1 Related Documents . 1 Regulatory Information . 1 Contacting SIGMA Technical Support . 1 Conventions . 2 Summary of Warnings and Cautions . 2 System Components . 11 Spectrum Pump Illustrations . 12 Hardware Labeling . 13 Battery Compatibility . 14 Setting Up the Pump . 15 Unpack the Pump . 15 AC Power Adaptor . 16 Cleaning the Power Adaptor . 16 Connecting the Power Adaptor . 16 Removing the Power Adaptor . 17 Charging the Battery . 17 Configuring User Options . 18 User Options . 18 Operational Overview . 21 Starting a New Infusion Using the Dose Error Reduction System (DERS) . 24 Starting a New Infusion using the BASIC Mode . 25 Secondary Infusions . 26 Preparing the Pump and IV Sets . 27 Loading an IV Set . 28 Unloading an IV Set . 30 Preparing the Pump for a Secondary Infusion . 30 Programming the Pump . 31 Infusion Programming Modes: . 32 Keys Used to Program and Operate the Pump . -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -

PFIZER INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2015 o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-3619 PFIZER INC. (Exact name of registrant as specified in its charter) Delaware 13-5315170 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification Number) 235 East 42nd Street New York, New York 10017-5755 (Address of principal executive offices) (Zip Code) (212) 733-2323 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Common Stock, $.05 par value New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
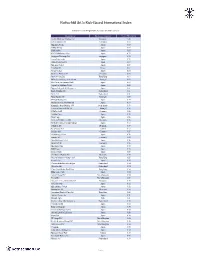
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Epidemiology/Health services research Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2021-002123 on 22 April 2021. Downloaded from Association between variability in body mass index and development of type 2 diabetes: Panasonic cohort study Hiroshi Okada ,1 Masahide Hamaguchi ,2 Momoko Habu,1 Kazushiro Kurogi,3 Hiroaki Murata,4 Masato Ito,3 Michiaki Fukui2 To cite: Okada H, ABSTRACT Hamaguchi M, Habu M, Introduction Contrasting results have been reported for Significance of this study et al. Association between the association between the variability in body weight variability in body mass index and development of diabetes. In the present study, we What is already known about this subject? and development of type 2 evaluated the association between the variability in body ► Obesity or body weight gain accelerate the develop- diabetes: Panasonic cohort ment of diabetes. study. BMJ Open Diab Res Care mass index (BMI) and development of type 2 diabetes in 19 412 Japanese participants without obesity and without ► The association between the variability in body 2021;9:e002123. doi:10.1136/ weight and development of diabetes is controversial. bmjdrc-2021-002123 body weight gain or loss during the study period. Research design and methods We recorded body weight What are the new findings? of the participants consecutively each year in Panasonic ► The risk for developing diabetes increased by 11.1% Received 9 January 2021 Corporation, Osaka, Japan from 2008 to 2014 to evaluate Revised 26 February 2021 per 1% increase in coefficient of variation of body the variability of BMI. The participants with obesity (BMI mass index (BMI). -

DCAT MEMBER COMPANY MEETING LOCATOR V. 6 the BENJAMIN Sri Krishna Pharmaceuticals Ltd
DCAT MEMBER COMPANY MEETING LOCATOR v. 6 THE BENJAMIN Sri Krishna Pharmaceuticals Ltd. Zydus Pharmaceuticals (USA) Inc. Amino Chemicals Ltd. Apogee Pharma, Inc. INTERCONTINENTAL BARCLAY C2 PHARMA AbbVie* Calyx Chemicals & Pharmaceuticals Ltd. ACIC Pharmaceuticals Inc. ChemCon GmbH Advitech SA Concord Biotech Limited Amneal Pharmaceuticals LLC Dipharma Francis Srl ALP Pharm DSM Sinochem Pharmaceuticals AMRI Emergent BioSolutions Asymchem Inc. F.I.S. - Fabbrica Italiana Sintetici S.p.A. Capsugel, Now a Lonza Company* Indena S.p.A. CBC AMERICAS Corp. Jost Chemical Co. CellMark USA, LLC Jubilant Pharma Charioteer Pharmaceutical Co., Ltd., Zhejiang PharmSource, A GlobalData Company Chemical and Pharmaceutical Solutions PolyPeptide Group Chiral Quest Corp. ROHNER Inc. Croda, Inc. SST Corporation DFE Pharma HOTEL 48LEX DPL-US EQ Esteve AB BioTechnologies, Inc. Evonik Corporation AGC Biologics FAREVA SA AiPing Pharmaceutical, Inc. Flavine North America, Inc. Almac Formosa Laboratories, Inc. Aptuit LLC Grifols International S.A. AZAD Fine Chemicals Ltd. Hainan Poly Pharm. Co., Ltd. Cambridge Isotope Laboratories, Inc. Harris Pharmaceutical Groupe Parima Helm AG Navin Fluorine International Limited Hetero USA, Inc. Qualicaps, Inc. Hikal, Ltd. RC2 Pharma Connect LLC Interchem Corporation Recipharm Inventia Healthcare PVT LTD Recro Gainesville LLC Johnson Matthey Reed-Lane, Inc. Kingchem Life Science LLC Sancilio Pharmaceuticals Company, Inc. Please note: Some DCAT member companies have requested not to be listed in the locator. (*) indicates member companies with Business Meeting Spaces in more than one hotel. INTERCONTINENTAL BARCLAY CONT'D LOTTE NY PALACE Legacy Pharmaceutical Packaging AbbVie* Lonza AG* Acella Pharmaceuticals Neuland Laboratories Ltd. Alcami Corporation Orion Group Alembic Pharmaceuticals Limited Par Pharmaceutical, Inc. Aphena Pharma Solutions PCI Pharma Services Aptar Pharma PHF SA Aristo Pharma Polymed Therapeutics, Inc. -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1.