Compositional Evolution of the Upper Continental Crust Through Time, As Constrained by Ancient Glacial Diamictites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Timeline of Natural History
Timeline of natural history This timeline of natural history summarizes significant geological and Life timeline Ice Ages biological events from the formation of the 0 — Primates Quater nary Flowers ←Earliest apes Earth to the arrival of modern humans. P Birds h Mammals – Plants Dinosaurs Times are listed in millions of years, or Karo o a n ← Andean Tetrapoda megaanni (Ma). -50 0 — e Arthropods Molluscs r ←Cambrian explosion o ← Cryoge nian Ediacara biota – z ←Earliest animals o ←Earliest plants i Multicellular -1000 — c Contents life ←Sexual reproduction Dating of the Geologic record – P r The earliest Solar System -1500 — o t Precambrian Supereon – e r Eukaryotes Hadean Eon o -2000 — z o Archean Eon i Huron ian – c Eoarchean Era ←Oxygen crisis Paleoarchean Era -2500 — ←Atmospheric oxygen Mesoarchean Era – Photosynthesis Neoarchean Era Pong ola Proterozoic Eon -3000 — A r Paleoproterozoic Era c – h Siderian Period e a Rhyacian Period -3500 — n ←Earliest oxygen Orosirian Period Single-celled – life Statherian Period -4000 — ←Earliest life Mesoproterozoic Era H Calymmian Period a water – d e Ectasian Period a ←Earliest water Stenian Period -4500 — n ←Earth (−4540) (million years ago) Clickable Neoproterozoic Era ( Tonian Period Cryogenian Period Ediacaran Period Phanerozoic Eon Paleozoic Era Cambrian Period Ordovician Period Silurian Period Devonian Period Carboniferous Period Permian Period Mesozoic Era Triassic Period Jurassic Period Cretaceous Period Cenozoic Era Paleogene Period Neogene Period Quaternary Period Etymology of period names References See also External links Dating of the Geologic record The Geologic record is the strata (layers) of rock in the planet's crust and the science of geology is much concerned with the age and origin of all rocks to determine the history and formation of Earth and to understand the forces that have acted upon it. -

Neoproterozoic to Cambrian Palaeoclimatic Events in Southwestern Gondwana$
CHAPTER 11.1 Neoproterozoic to Cambrian Palaeoclimatic Events in Southwestern Gondwana$ A.J. Kaufman1, A.N. Sial2, H.E. Frimmel3 and A. Misi4 Contents 11.1.1. Constructing a Global Record of Neoproterozoic Palaeoclimatic Variations 369 11.1.2. Age Constraints for Cryogenian Glacial Deposits in Southwestern Gondwana 371 11.1.2.1. Sturtian 371 11.1.2.2. Marinoan 372 11.1.2.3. Gaskiers 372 11.1.3. Chemostratigraphic Records of Palaeoclimatic Events in Southwestern Gondwana 373 11.1.3.1. Carbon isotopes 373 11.1.3.2. Strontium isotopes 373 11.1.3.3. Isotopic observations of pre-Sturtian (?) ice ages in southwestern Gondwana 374 11.1.3.4. Lithologic and isotopic observations of Sturtian ice ages in southwestern Gondwana 376 11.1.3.5. Lithologic and isotopic observations of Marinoan ice ages in southwestern Gondwana 377 11.1.3.6. Lithologic and isotopic observations of Gaskiers ice ages in southwestern Gondwana 381 11.1.3.7. Palaeoclimatic change at the Ediacaran-Cambrian boundary and beyond 382 11.1.4. A Synthesis of the Palaeoclimatic Puzzle from Southwestern Gondwana 383 11.1.4.1. The Hu¨ttenberg positive carbon isotope anomaly 383 11.1.4.2. Strontium isotope correlations of cap carbonates in southwestern Gondwana 386 11.1.5. Conclusions 388 Acknowledgements 388 11.1.1. Constructing a Global Record of Neoproterozoic Palaeoclimatic Variations Since publication of the ‘Snowball Earth’ hypothesis 2 initially by Kirschvink (1992a) based on an apparently robust equatorial palaeolatitude for glacial strata in the Neoproterozoic Elatina Formation of Australia, and later by Hoffman et al. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

765–740 Ma Kansuki-Mwashya Platform Succession in the Tenke-Fungurume Mining District, Democratic Republic of the Congo
GEOLOGICA BELGICA (2020) 23/1-2: 69-85 Sedimentary evolution and stratigraphy of the ~765–740 Ma Kansuki-Mwashya platform succession in the Tenke-Fungurume Mining District, Democratic Republic of the Congo PASCAL MAMBWE1,2, FRANCK DELPOMDOR3*, SÉBASTIEN LAVOIE2, PHILIPPE MUKONKI4, JACQUES BATUMIKE4,5 & PHILIPPE MUCHEZ1 1 KU Leuven, Department of Earth & Environmental Sciences, Celestijnenlaan 200E, B-3001 Leuven, Belgium; mambwegeo@ gmail.com, [email protected]. 2 Tenke Fungurume Mining S.A., Department of Exploration Geology, Route de l’Aéroport, Bâtiment TFM, Commune Annexe, Lubumbashi, Democratic Republic of the Congo; [email protected]. 3 Illinois State Geological Survey, University of Illinois at Urbana-Champaign, 615 E. Peabody Dr, Champaign, IL 61820, United States of America; [email protected]. 4 University of Lubumbashi, Department of Geology, 14 Kassapa Road, Lubumbashi, Democratic Republic of the Congo; [email protected], [email protected]. 5 ARC Centre of Excellence for Core to Crust Fluid Systems (CCFS) and GEMOC, Department of Earth and Planetary Sciences, Macquarie University, NSW 2109, Australia. * corresponding author. ABSTRACT. The origin of the Mwashya Conglomerate at the base of the Mwashya Subgroup in the Lufilian Belt is uncertain since it is considered as either a tectonic or as a sedimentary breccia. At Tenke Fungurume Mining District (TFMD) in the Democratic Republic of the Congo, the Mwashya Conglomerate is marked by an iron-bearing polymictic conglomerate embedded between the Kansuki and Kamoya formations. In this paper, the Kansuki-Mwashya platform succession at TFMD was investigated to shed light on the origin of this conglomerate, the depositional evolution and the tectonostratigraphic framework of the platform. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
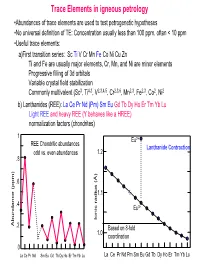
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand?
The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand? Shun-ichiro Karato, David Bercovici, Garrett Leahy, Guillaume Richard and Zhicheng Jing Yale University, Department of Geology and Geophysics, New Haven, CT 06520 Materials circulation in Earth’s mantle will be modified if partial melting occurs in the transition zone. Melting in the transition zone is plausible if a significant amount of incompatible components is present in Earth’s mantle. We review the experimental data on melting and melt density and conclude that melting is likely under a broad range of conditions, although conditions for dense melt are more limited. Current geochemical models of Earth suggest the presence of relatively dense incompatible components such as K2O and we conclude that a dense melt is likely formed when the fraction of water is small. Models have been developed to understand the structure of a melt layer and the circulation of melt and vola- tiles. The model suggests a relatively thin melt-rich layer that can be entrained by downwelling current to maintain “steady-state” structure. If deep mantle melting occurs with a small melt fraction, highly incompatible elements including hydro- gen, helium and argon are sequestered without much effect on more compatible elements. This provides a natural explanation for many paradoxes including (i) the apparent discrepancy between whole mantle convection suggested from geophysical observations and the presence of long-lived large reservoirs suggested by geochemical observations, (ii) the helium/heat flow paradox and (iii) the argon paradox. Geophysical observations are reviewed including electrical conductivity and anomalies in seismic wave velocities to test the model and some future direc- tions to refine the model are discussed. -

28Th Debeers Alex. Du Toit Memorial Lecture, 2004. on Cryogenian (Neoproterozoic) Ice-Sheet Dynamics and the Limitations of the Glacial Sedimentary Record Paul F
PAUL F. HOFFMAN 557 28th DeBeers Alex. Du Toit Memorial Lecture, 2004. On Cryogenian (Neoproterozoic) ice-sheet dynamics and the limitations of the glacial sedimentary record Paul F. Hoffman Department of Earth and Planetary Sciences Harvard University, 20 Oxford Street, Cambridge, MA 02138, U.S.A. e-mail: [email protected] © 2005 December Geological Society of South Africa ABSTRACT The snowball earth hypothesis is a unified theory accounting for the global distribution of Cryogenian (roughly 720 to 635 Ma) glacial and glacial marine deposits, their global synchroneity demonstrated by chemostratigraphy, and their close association with thick carbonate strata and sedimentary iron deposits (banded iron formation) in certain areas. It postulates that on two separate occasions, around 710 and 640 Ma, the ocean froze over from pole to pole for long periods (i.e., millions of years). The postulate has been widely criticized as being incompatible with the glacial sedimentary record indicating the former existence of fast-moving wet-base ice and open proglacial waters. The younger Cryogenian glaciation in northern Namibia presents an excellent opportunity to investigate the sedimentary record. The area was then a vast shallow-water carbonate platform situated in the tropics or subtropics. The platform had a sharply- defined southern edge, beyond which a stratigraphically tapered foreslope wedge was descending into deep waters of the northern Damara extended terrain. The platform and foreslope were undergoing broad regional subsidence with no local structural deformation at the time of the younger glaciation. The Fransfontein Ridge (a present physiographic feature) is a simple homocline exposing a continuous 60-km-long section of the foreslope wedge. -

Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer 2. 1Department Of
47th Lunar! and Planetary Science Conference (2016) 1058.pdf Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer2. 1Department of Earth and Environmental Science, New Mexico Tech, Socorro, New Mexico 87801, 2 Institute of Meteoritics, Department of Earth and Planetary Science, University of New Mexico, Albuquerque, New Mexico 87131, 2 NASA Johnson Space Center, Houston, TX 77058. Introduction: Because ratios of elements with group centered near Earth’s primitive mantle (PM) similar incompatibilities do not change much with composition (Fig. 2). This distribution is similar to degree of melting or fractional crystallization in the the distribution of most mare basalts from the Moon mantles of silicate planets, they are useful in sampled by the Apollo missions, except for the high- characterizing compositions of mantle sources of Ti basalts from Apollo 11 and 17 both of which are basalts that have not interacted with continental crust enriched in Nb. The primitive mantle grouping is [1,2]. Using Nb/Th and Zr/Nb ratios in young especially evident for Apollo 11, 12 and 15 basalts in oceanic basalts, three mantle domains can be which samples cluster tightly around primitive identified [3]: enriched (EM), depleted (DM), and mantle (PM) composition on Zr/Nb-Nb/Th, Th/Yb- hydrated mantle (HM) (Fig. 1). DM is characterized Nb/Yb and La/Sm-Nb/Th diagrams (Fig. 3). Very by high values of both ratios (Nb/Th > 8; Zr/Nb > 20) low-Ti green volcanic glasses (and some due to Th depletion relative to Nb, and to Nb intermediate-Ti yellow volcanic glasses) plot in a depletion relative to Zr. -

Insights Into Chemical Weathering of the Upper Continental Crust from the Geochemistry of Ancient Glacial Diamictites
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 176 (2016) 96–117 www.elsevier.com/locate/gca Insights into chemical weathering of the upper continental crust from the geochemistry of ancient glacial diamictites Su Li a,b,⇑, Richard M. Gaschnig b,2, Roberta L. Rudnick b,1 a School of Geosciences, China University of Petroleum (East China), Qingdao 266555, China b Geology Department, University of Maryland, College Park, MD 20742-421, USA Received 12 June 2015; accepted in revised form 12 December 2015; Available online 21 December 2015 Abstract Glacial diamictites, with ages ranging from 2900 to 0.01 Ma, record the changing composition of the upper continental crust through time (Gaschnig et al., 2014). Li concentrations and isotopic compositions, combined with Pb isotopic compo- sitions, chemical index of alteration (CIA) values and relative Sr concentrations are used here to assess the degree of chemical weathering recorded in these deposits and the origin of this signature. The d7Li values of most of the diamictites (ranging from À3.9 to +3.5) are lower than those of mantle-derived basalts (+3.7 ± 2, 2r), and the low d7Li values are generally accompa- nied by high CIA and low Sr/Sr* values (or Sr depletion factor, Sr/Sr* = Sr/(Ce*Nd)0.5), reflecting a weathering signature that may have derived from pre-depositional, syn-depositional, and/or post-depositional weathering processes. Profiles through three glacial diamictites with relatively high CIA (a fresh road cut of the Neoproterozoic Nantuo Formation (CIA = 62– 69), and drill cores through the Paleoproterozoic Timeball Hill (CIA = 66–75) and Duitschland Formations (CIA = 84– 91)) do not show evidence of significant post-depositional weathering. -

Durham Research Online
Durham Research Online Deposited in DRO: 14 March 2018 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Namur, Olivier and Humphreys, Madeleine C.S. (2018) 'Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland.', Journal of petrology., 59 (3). pp. 387-418. Further information on publisher's website: https://doi.org/10.1093/petrology/egy032 Publisher's copyright statement: This is a pre-copyedited, author-produced version of an article accepted for publication in Journal Of Petrology following peer review. The version of record Namur, Olivier Humphreys, Madeleine C.S. (2018). Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland. Journal of Petrology is available online at: https://doi.org/10.1093/petrology/egy032. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Trace element constraints on the differentiation and crystal mush solidification in the Skaergaard intrusion, Greenland Olivier Namur1*, Madeleine C.S.