Epidural Steroid Injection with Supplemental Oral Eplerenone for Low Back Pain: a Prospective, Double Blind Randomized Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"Macrolides"? Classify Each Drug in This Chapter As a Macrolide Or Azalide, and As an Antibiotic Or Semi-Synthetic Derivative
STUDY GUIDE THE MACROLIDE/AZALIDE ANTIMICROBIAL AGENTS 1. Why are these antibiotics derivatives called "macrolides"? Classify each drug in this chapter as a macrolide or azalide, and as an antibiotic or semi-synthetic derivative. 2. What are the key structural differences between erythromycin, clarithromycin, azithromycin and dirithromycin? 3. Generally how does spiramycin and josamycin differ in structure from the commercial macrolides/azalides (2 reasons)? 4. What are the “ketolides and how do they differ in structure from the commercial macrolides? 5. What is the biosynthetic source of erythromycin? What is the lactone moiety of erythromycin called? What sugar moieties are present and what are their properties? 6. What hydrolysis product forms from erythromycin in aqueous acid or base? What is the significance of this reaction? Can it occur with other macrolides? 7. When does the “intramolecular cyclization” reaction occur with erythromycin? What is it's significance (two reasons) and how does it occur? Which functional groups are important for this reaction? 8. What salt forms of erythromycin are available? Which are water soluble? Which are water-insoluble? How is each salt form formulated and used (oral or parenteral)? 9. What ester and ester salt derivatives of erythromycin are available? What is the estolate? How is each salt form formulated and used (oral or parenteral)? What are the advantages of these esters dosage forms? 10. How does clarithromycin differ in structure from erythromycin? Why was this macrolide developed (the role of the 6-methoxy)? 11. How does azithromycin differ in structure from erythromycin? Why was this macrolide developed? 12. How does dirithromycin differ in structure from erythromycin? Why was this macrolide developed? What is the active form of this prodrug? 13. -

35 Cyproterone Acetate and Ethinyl Estradiol Tablets 2 Mg/0
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrCYESTRA®-35 cyproterone acetate and ethinyl estradiol tablets 2 mg/0.035 mg THERAPEUTIC CLASSIFICATION Acne Therapy Paladin Labs Inc. Date of Preparation: 100 Alexis Nihon Blvd, Suite 600 January 17, 2019 St-Laurent, Quebec H4M 2P2 Version: 6.0 Control # 223341 _____________________________________________________________________________________________ CYESTRA-35 Product Monograph Page 1 of 48 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION ....................................................................... 3 SUMMARY PRODUCT INFORMATION ............................................................................................. 3 INDICATION AND CLINICAL USE ..................................................................................................... 3 CONTRAINDICATIONS ........................................................................................................................ 3 WARNINGS AND PRECAUTIONS ....................................................................................................... 4 ADVERSE REACTIONS ....................................................................................................................... 13 DRUG INTERACTIONS ....................................................................................................................... 16 DOSAGE AND ADMINISTRATION ................................................................................................ 20 OVERDOSAGE .................................................................................................................................... -

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -

Portia®(Levonorgestrel and Ethinyl Estradiol Tablets USP)
PORTIA- levonorgestrel and ethinyl estradiol Teva Pharmaceuticals USA, Inc. ---------- Portia® (levonorgestrel and ethinyl estradiol tablets USP) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Portia® (levonorgestrel and ethinyl estradiol tablets USP) consists of 21 pink active tablets, each containing 0.15 mg of levonorgestrel, USP, (18, 19-Dinorpregn-4-en-20-yn- 3-one, 13-ethyl-17-hydroxy-, (17α)-(−)-), a totally synthetic progestogen, and 0.03 mg of ethinyl estradiol, USP, (19-nor-17α-pregna-1,3,5 (10)-trien-20-yne-3,17-diol), and 7 white inert tablets. The inactive ingredients in the pink active tablets include anhydrous lactose, hypromellose, magnesium stearate, and microcrystalline cellulose. The ingredients in the film-coating include FD&C blue no. 1 aluminum lake, FD&C red no. 40 aluminum lake, hypromellose, polyethylene glycol, polysorbate 80, and titanium dioxide. Each white inert tablet contains anhydrous lactose, hypromellose, magnesium stearate, and microcrystalline cellulose. The structural formulas are as follows: Levonorgestrel, USP C21H28O2 M.W. 312.45 Ethinyl Estradiol, USP C20H24O2 M.W. 296.40 CLINICAL PHARMACOLOGY Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation). INDICATIONS AND USAGE Oral contraceptives are indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. Oral contraceptives are highly effective. -

FDA Approved Medications Part 3: Review of Individual Medications Part 4: Additional Board-Pertinent Information
Dr. Jack’s MedQuik Guide A Psychotropic Medication Guide for Board Exam Preparation Free Additional Board Exam Preparation Resources www.BeatTheBoards.com • 877-225-8384 Dr. Jack’s MedQuik Guide Table of Contents Part 1: Medications FDA Approved In 2009-2010 Part 2: List of Psychiatric Disorders With Their FDA Approved Medications Part 3: Review of Individual Medications Part 4: Additional Board-Pertinent Information Copyright Notice Copyright © 2007-2010 American Physician Institute for Advanced Professional Studies, LLC. All rights reserved. This manuscript may not be transmitted, copied, reprinted, in whole or in part, without the express written permission of the copyright holder. Requests for permission or further information should be addressed to Jack Krasuski at: [email protected] or American Physician Institute for Advanced Professional Studies, LLC, 125 Windsor Dr., Suite 111, Oak Brook, IL 60523 Disclaimer Notice This publication is designed to provide general educational advice. It is provided to the reader with the understanding that Jack Krasuski and American Physician Institute for Advanced Professional Studies LLC are not rendering medical services. This guide is designed to aid physicians in exam preparation and is not to be considered medical advice for guiding medical care of patients. If medical or other expert assistance is required, the services of a medical or other consultant should be obtained. The author and publisher disclaim any liability arising directly or indirectly from the use of this book. -
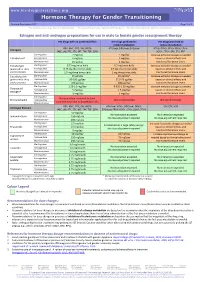
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -

Divestra Leaflet
• Known or suspected estrogen-dependent neoplasia Drugs which may decrease the therapeutic effect of Cyproterone acetate+Ethinyl Pregnancy and Breastfeeding • Undiagnosed abnormal vaginal bleeding Combination of Cyproterone acetate and Ethinyl estradiol is contraindicated during estradiol and increase the incidence of breakthrough bleeding • Any ocular lesion arising from ophthalmic vascular disease, such as partial or complete pregnancy and breastfeeding. loss of vision or defect in visual fields Effects on ability to drive and use machines • Concomitant use with other Estrogen+Progestogen combinations or estrogens or Unknown progestogens alone • When pregnancy is suspected or diagnosed Adverse Reactions • Severe diabetes with vascular changes Common adverse reactions includes headaches, nausea, abdominal pain, weight gain, • A history of otosclerosis with deterioration during pregnancy depressed or altered mood and breast pain or tenderness. • Hypersensitivity to this drug or to any ingredient in the formulation or component of the Uncommon adverse reactions include vomiting, diarrhea, fluid retention and migraine. container. Overdose (Cyproterone acetate + Warnings and Precautions There is no antidote and treatment should be symptomatic. Ethinyl estradiol) Discontinue Cyproterone acetate+Ethinyl estradiol tablets at the earliest manifestation of the following: PHARMACOLOGICAL PROPERTIES • Thromboembolic and Cardiovascular Disorders such as thrombophlebitis, pulmonary Pharmacotherapeutic group: Sex hormones and modulators of the genital system, QUALITATIVE AND QUANTITATIVE COMPOSITION embolism, cerebrovascular disorders, myocardial ischemia, mesenteric thrombosis, and anti-androgens and estrogens. ATC code: G03HB01 Each film-coated tablet contains: retinal thrombosis. Cyproterone acetate (Ph.Eur.)………...2 mg • Conditions that predispose to Venous Stasis and to Vascular Thrombosis (eg. Mechanism of action Ethinyl estradiol (USP)……………...0.035 mg immobilization after accidents or confinement to bed during long-term illness). -

Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Details Generic Name Indinavir Trade Name Crixivan® Class Protease Inhibitor Molecular Weight 711.88 (as sulphate) Structure HO OH N N H H SO N N 2 4 O HN O H3C CH3 H3C Summary of Key Pharmacokinetic Parameters Plasma half life 1.8 ± 0.4 h Cmax 8.97 ± 2.87 µg/ml (800 mg every 8 hours) Cmin 0.146 µg/ml (800 mg every 8 hours) AUC 21.82 ± 8.11 µg/ml.h (800 mg every 8 hours) Bioavailability Approx 65% Absorption Administration of indinavir with a meal high in calories, fat, and protein resulted in a blunted and reduced absorption with an approximate 80 % reduction in AUC and an 86 % reduction in Cmax. Administration with light meals (e.g., dry toast with jam or fruit conserve, apple juice, and coffee with skimmed or fat–free milk and sugar or corn flakes, skimmed or fat–free milk and sugar) resulted in plasma concentrations comparable to the corresponding fasted values. Protein Binding ~60% Volume of Distribution ~1.74 L/kg [1] CSF:Plasma ratio 0.14 [2] Semen:Plasma ratio 1.9 [2] Renal Clearance <20% as unchanged drug Renal Impairment Safety in patients with impaired renal function has not been studied; less than 20% of indinavir is excreted in the urine as unchanged drug or metabolites. Hepatic Impairment Safety and efficacy of indinavir has not been established in patients with significant underlying liver disorders and should be used with caution. -

Drugs Interfering with the Metabolism of Tacrolimus (FK506)
BLOOD SCIENCES DEPARTMENT OF CLINICAL BIOCHEMISTRY Title of Document: Drugs Interfering with the metabolism of tacrolimus (FK506) Q Pulse Reference No: BS/CB/DCB/TOX/4 Version NO: 6 Authoriser: P Beresford Page 1 of 3 Drugs Interfering with the Metabolism of Tacrolimus (FK506) Introduction Tacrolimus (FK506) is an immunosuppressant drug. The purpose of this protocol is to highlight drug interactions in the metabolism of tacrolimus (FK506) Systemically available tacrolimus is metabolised by hepatic CYP3A4. There is also evidence of gastrointestinal metabolism by CYP3A4 in the intestinal wall. Concomitant use of medicinal products or herbal remedies known to inhibit or induce CYP3A4 may affect the metabolism of tacrolimus and thereby increase or decrease tacrolimus blood levels. It is therefore recommended to monitor tacrolimus blood levels whenever substances which have the potential to alter CYP3A metabolism are used concomitantly and to adjust the tacrolimus dose as appropriate in order to maintain similar tacrolimus exposure Inhibitors of metabolism Clinically the following substances have been shown to increase tacrolimus blood levels: Strong interactions have been observed with antifungal agents such as ketoconazole, fluconazole, itraconazole and voriconazole, the macrolide antibiotic erythromycin or HIV protease inhibitors (e.g. ritonavir). Concomitant use of these substances may require decreased tacrolimus doses in nearly all patients. Weaker interactions have been observed with clotrimazole, clarithromycin, josamycin, nifedipine, nicardipine, diltiazem, verapamil, danazol, ethinylestradiol, omeprazole and nefazodone. In vitro the following substances have been shown to be potential inhibitors of tacrolimus metabolism: bromocriptine, cortisone, dapsone, ergotamine, gestodene, lidocaine, mephenytoin, miconazole, midazolam, nilvadipine, norethisterone, quinidine, tamoxifen, troleandomycin. Grapefruit juice has been reported to increase the blood level of tacrolimus and should therefore be avoided. -

Drug-Drug Interaction Between Protease Inhibitors and Statins and Proton Pump Inhibitors
Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Item Type text; Electronic Report Authors Orido, Charles; McKinnon, Samantha Publisher The University of Arizona. Rights Copyright © is held by the author. Download date 01/10/2021 01:48:07 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/636245 Group 47 :Orido/Samantha 1 Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Course Title: PhPr 862 Date: April 3, 2019 Faculty Advisor: Dr. Dan Malone Students: Charles Orido, Samantha McKinnon Pharm.D. Candidates, Class of 2019 Group 47 :Orido/Samantha 2 Objective The purpose of this article is to provide a systematic review of the pharmacokinetic and clinical data on drug-drug interactions between protease inhibitors (PIs) and statins, atazanavir and proton pump inhibitors (PPIs)and their clinical relevance. Methods A literature search was performed using Medline, EMBASE and google scholar, abstracts from 1970 to 2019 of major conferences were searched and FDA drug information package inserts of the manufacturer of every currently available PI was looked at. All data was summarized and verified by at least two investigators. Results A total of 246 references were identified, 8 of which were studies of pharmacokinetic and pharmacodynamics interactions between simvastatin, lovastatin and protease inhibitors and an additional 7 articles that provided pharmacokinetic of proton pump inhibitors and Atazanavir. Conclusions Protease inhibitors increases the AUC and Cmax of simvastatin by approximately 500% and 517% respectively. Therefore, simvastatin and Lovastatin are not recommended for a co-administration with a protease inhibitor. -

Crixivan (Indinavir Sulfate)
XXXXXXX CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN* (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, and 400 mg of indinavir (corresponding to 125, 250, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin and titanium dioxide. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2 (phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4 • H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. MICROBIOLOGY Mechanism of Action: HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Antiretroviral Activity In Vitro: The in vitro activity of indinavir was assessed in cell lines of lymphoblastic and monocytic origin and in peripheral blood lymphocytes. HIV-1 variants used to infect the different cell types include laboratory-adapted variants, primary clinical isolates and clinical isolates resistant to nucleoside analogue and nonnucleoside inhibitors of the HIV-1 reverse transcriptase.