(PBS) Listing of Medicines: the Case of Pregabalin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brisbane Native Plants by Suburb
INDEX - BRISBANE SUBURBS SPECIES LIST Acacia Ridge. ...........15 Chelmer ...................14 Hamilton. .................10 Mayne. .................25 Pullenvale............... 22 Toowong ....................46 Albion .......................25 Chermside West .11 Hawthorne................. 7 McDowall. ..............6 Torwood .....................47 Alderley ....................45 Clayfield ..................14 Heathwood.... 34. Meeandah.............. 2 Queensport ............32 Trinder Park ...............32 Algester.................... 15 Coopers Plains........32 Hemmant. .................32 Merthyr .................7 Annerley ...................32 Coorparoo ................3 Hendra. .................10 Middle Park .........19 Rainworth. ..............47 Underwood. ................41 Anstead ....................17 Corinda. ..................14 Herston ....................5 Milton ...................46 Ransome. ................32 Upper Brookfield .......23 Archerfield ...............32 Highgate Hill. ........43 Mitchelton ...........45 Red Hill.................... 43 Upper Mt gravatt. .......15 Ascot. .......................36 Darra .......................33 Hill End ..................45 Moggill. .................20 Richlands ................34 Ashgrove. ................26 Deagon ....................2 Holland Park........... 3 Moorooka. ............32 River Hills................ 19 Virginia ........................31 Aspley ......................31 Doboy ......................2 Morningside. .........3 Robertson ................42 Auchenflower -
Inner Brisbane Heritage Walk/Drive Booklet
Engineering Heritage Inner Brisbane A Walk / Drive Tour Engineers Australia Queensland Division National Library of Australia Cataloguing- in-Publication entry Title: Engineering heritage inner Brisbane: a walk / drive tour / Engineering Heritage Queensland. Edition: Revised second edition. ISBN: 9780646561684 (paperback) Notes: Includes bibliographical references. Subjects: Brisbane (Qld.)--Guidebooks. Brisbane (Qld.)--Buildings, structures, etc.--Guidebooks. Brisbane (Qld.)--History. Other Creators/Contributors: Engineers Australia. Queensland Division. Dewey Number: 919.43104 Revised and reprinted 2015 Chelmer Office Services 5/10 Central Avenue Graceville Q 4075 Disclaimer: The information in this publication has been created with all due care, however no warranty is given that this publication is free from error or omission or that the information is the most up-to-date available. In addition, the publication contains references and links to other publications and web sites over which Engineers Australia has no responsibility or control. You should rely on your own enquiries as to the correctness of the contents of the publication or of any of the references and links. Accordingly Engineers Australia and its servants and agents expressly disclaim liability for any act done or omission made on the information contained in the publication and any consequences of any such act or omission. Acknowledgements Engineers Australia, Queensland Division acknowledged the input to the first edition of this publication in 2001 by historical archaeologist Kay Brown for research and text development, historian Heather Harper of the Brisbane City Council Heritage Unit for patience and assistance particularly with the map, the Brisbane City Council for its generous local history grant and for access to and use of its BIMAP facility, the Queensland Maritime Museum Association, the Queensland Museum and the John Oxley Library for permission to reproduce the photographs, and to the late Robin Black and Robyn Black for loan of the pen and ink drawing of the coal wharf. -

Woolloongabba to the University of Queensland (UQ)
BRACKEN RDGE RD BRACKEN RDGE RD WARNER GMPE RD KREMW RD BRACKEN RIDE LD N RD DENHAM ST SANDATE S PNE RD BARD ST PHLLPS ST S PNE RD DEAN SHRNCLIE BRENDALE EATONS CRO NRRS RD SSI NG LNKFELD RD GMPE ARTERAL RD R D S PNE RD TELEGRAPH RD G Y M P I E R D G ATEW AY MOTORWAY D R E K M LE LACE RD EATNS RDLE RD RGHAN RD HILL BEAMS RD ALBAN GMPE RD CREEK CARSELDINE BNDALL TAIUM DRVLLE RD ITIBBN BEAMS RD ALB AN Y C RE GRAHAM RD HANDFRD RD NUDEE EK KENG RD RD BEACH BRIDEMAN GA TEW AY DWNS MOT ORW LLMERE RD AY ILLMERE SANDGATE RD LLMERE RD KRB RD RBNSN RD W BECKETT RD ASLE BUNA HRN RD RBNSN RD W NUDEE MURPH RD EEBUN TRACK KER JIN ELLSN RD VIRINIA BAN MAUNDRELLCHERMSIDE TCE MCDWALL WEST K ST VNCENTS RD ITTY HAMLTN RD H O L A D NEWMAN RD TUFNELL RD W N K O BUNA RD D NRTHATE R T R H E R N MAN AVE EARNSHAW RD R TRUTS RD D D HAMLTN RD R CHERMSIDE E TMBUL RD T A G NUDGEE RD RDE RD D N A S WAVELL EVERTN HEIHTS G FLCKTN ST Y HILLS M RDE RD ARPRT DR P I C E A PATRCKS RD M D STARD R E R D L MELTN RD IA EVERTN S T HEIHTS A V U E PARK O R T ARANA DAWSN PDE TMBUL S FELSTEAD ST HILLS P MRETN DR I N SHAW RD E NUNDAH R REDWD ST D BUCKLAND RD KEDRN APPLEB RD WDDP ST WEBSTER RD MITCHELTN KEERRA STAFFRD RD ARPRT DR RDN D PARK R RO K SE ST R PA T S D N A GERLER RD H S THSTLE ST T LTTN S BRADSHAW ST LMANDRA DR E LMANDRA DR ATHRNE G D I RAMNT RD R RANE B S O U T H R D E RT R PO BNNE AVE FULLER ST STNELEGH ST N PRTCHARD ST C KTCHENER RD R D REL RD O D DAS RD R R YGAR S A M ST LUTWCHE D S M R N NUDGEE RD R D W O A K L F A S A E E D N D Y L D U Y R O G ALBN RD H A R D ASCT -

DNRM RTI DL Release
Author: G. Swann File Number: 190 ~7 'I Woolloongabba Office South East Region Phone: 3224 7373 5 July2007 Rinker Australia Pty Ltd PO Box 1143 Milton Q 4067 Attention - Operations Manager - Dear Sir Information Notice - Renewal of Quarry Material Allocation Notice Number: 100740 This information notice is given in accordance with section 289 (5) ofthe Water Act 2000 ("the Act") in respect of the decision on the above application.Release Background Matters DL This allocation refers to the extraction of quarry materials from the Brisbane River at a location known as Summerville's Land (Left Bank). Decision RTI The Department ofNatural Resources and Water delegates officers to exercise the power of the chief executive to make decisions about applications for a renewal of a Quarry Material - Allocation. As a delegated officer of this Department, I have decided to grant with conditions the above application and provideDNRM the following information about my decision. This information notice is advice of my decision and the reasons for the decision. Copies of this information notice have been sent to all persons who made a properly made submission with respect to the application. Evidence Or Other Material On Which Findings Of Fact Were Made Renewal of this allocation has been issued for the same maximum extraction rate as previously issued. Level 3 Landcentre Cnr Main & Vulture Streets Woolloongabba Qld 4102 PO Box 1653 Coorparoo Queensland 4151 Australia Telephone + 61 7 32247373 Facsimile + 61 7 32242933 Website www.nrm.qld.gov.au 14-203 File H Page 1 of 42 Release Findings On Material Questions Of Fact • Renewal and transfer application made by Rinker Australia Pty Ltd which was received on 26 February 2007 with prescribed fee. -

Kangaroo Point M Apartments Kangaroo Point
DARK FINISHES KANGAROO POINT M APARTMENTS KANGAROO POINT Sophisticated living in a hotspot urban village. Location, design, quality and value: M Apartments put it all on the table for you at Kangaroo Point – transforming before our eyes into Brisbane’s most dynamic new urban environment. Close to the city, close to everything. The coolest places to meet and mingle, eat and shop, just a hop, skip and jump from transport, nightlife, parks and open spaces. In an area that mixes the old with the new and the unexpected, M Apartments radiate cool. Think flair plus functionality, ultra-smart aesthetics, space and comfort. Make your move and secure a vibrant slice of cosmopolitan life, in a locale primed for future growth. M Apartments, Kangaroo Point. 02 03 M APARTMENTS KANGAROO POINT A location brimming with energy and vitality. Kangaroo Point is booming and demand is rising, in this ultra-convenient and connected neighbourhood only 1.5km across the river from the CBD. Capturing the buzz of inner-city living and echoing Kangaroo Point’s revitalized contemporary edge, M Apartments will be the sought-after home for up and coming professionals, students at the nearby tertiary campuses, astute downsizers and investors with a keen eye for capital gains. It’s all here for the taking, in this one-of- a-kind location. Stroll, hop on a CityCat or ferry, catch the bus or drive the Clem7 cross-river tunnel. A new green bridge is planned, to link Kangaroo Point even more closely with the CBD. Plus, easy proximity to Woolloongabba, South Bank, Fortitude Valley, the clubs, pubs, bars, dining spots and designer stores puts Kangaroo Point clearly at the centre of Brisbane’s property radar. -

Looking at a Strengthening Market and How to Act
APARTMENT In this edition of Apartment Magazine, we look at why you should consider buying new and some of the hidden costs ISSUE 09 associated with purchasing a property. Our team also delve into the stats and report on the strengthening market in SUMMER 19/20 South East Queensland, and how Kangaroo Point is being rediscovered as a dominant player in the Brisbane market. LOOKING AT A STRENGTHENING MARKET AND HOW TO ACT NOW WHY PLACE PROJECTS FOR YOUR INVESTMENT? It’s a seamless approach with your investment, from purchase to management. Place Projects Property Management builds relationships starting with YOU. As our client, you will receive a high level of communication from experienced property managers. 07 3107 9223 We have a boutique portfolio and pay attention to maximise [email protected] your investment return. We focus on tenant selection and work Level 1, 33 Lytton Road hard to keep your investment producing for the long term. East Brisbane IS THERE ANY BENEFIT IN BUYING A NEW PROPERTY? APART FROM THE OBVIOUS AND DESIRABLE NEW FEEL AND LOOK, THERE ARE SEVERAL OTHER REASONS WHY BUYING A NEW PROPERTY CAN PAY OFF. Firstly, first home buyers have a huge benefit of receiving $15,000 cash if they purchase a new home for less than $750,000. Secondly, the chance of unforeseen repairs is lower for newer properties and may still be covered under warranty. Additionally, body corporate contributions can be considerably less than some older units that need to boost the WHY BUY amount in the sinking fund. There is also extra security in knowing that if you decide to rent out your new property that you will get a premium price compared to established properties. -

Register of Architects & Non Practising Architects
REGISTER OF ARCHITECTS & NON PRACTISING ARCHITECTS Copyright The Board of Architects of Queensland supports and encourages the dissemination and exchange of information. However, copyright protects this document. The Board of Architects of Queensland has no objection to this material being reproduced, made available online or electronically , provided it is for your personal, non-commercial use or use within your organisation; this material remains unaltered and the Board of Architects of Queensland is recognised as the owner. Enquiries should be addressed to: [email protected] Register As At 29 June 2021 In pursuance of the provision of section 102 of Architects Act 2002 the following copy of the Register of Architects and Non Practicing Architects is published for general information. Reg. No. Name Address Bus. Tel. No. Architects 5513 ABAS, Lawrence James Ahmad Gresley Abas 03 9017 4602 292 Victoria Street BRUNSWICK VIC 3056 Australia 4302 ABBETT, Kate Emmaline Wallacebrice Architecture Studio (07) 3129 5719 Suite 1, Level 5 80 Petrie Terrace Brisbane QLD 4000 Australia 5531 ABBOUD, Rana Rita BVN Architecture Pty Ltd 07 3852 2525 L4/ 12 Creek Street BRISBANE QLD 4000 Australia 4524 ABEL, Patricia Grace Elevation Architecture 07 3251 6900 5/3 Montpelier Road NEWSTEAD QLD 4006 Australia 0923 ABERNETHY, Raymond Eric Abernethy & Associates Architects 0409411940 7 Valentine Street TOOWONG QLD 4066 Australia 5224 ABOU MOGHDEB EL DEBES, GHDWoodhead 0403 400 954 Nibraz Jadaan Level 9, 145 Ann Street BRISBANE QLD 4000 Australia 4945 ABRAHAM, -

Brisbane City Plan, Appendix 2
Introduction ............................................................3 Planting Species Planning Scheme Policy .............167 Acid Sulfate Soil Planning Scheme Policy ................5 Small Lot Housing Consultation Planning Scheme Policy ................................................... 168a Air Quality Planning Scheme Policy ........................9 Telecommunication Towers Planning Scheme Airports Planning Scheme Policy ...........................23 Policy ..................................................................169 Assessment of Brothels Planning Scheme Transport, Access, Parking and Servicing Policy .................................................................. 24a Planning Scheme Policy ......................................173 Brisbane River Corridor Planning Scheme Transport and Traffic Facilities Planning Policy .................................................................. 24c Scheme Policy .....................................................225 Centre Concept Plans Planning Scheme Policy ......25 Zillmere Centre Master Plan Planning Scheme Policy .....................................................241 Commercial Character Building Register Planning Scheme Policy ........................................29 Commercial Impact Assessment Planning Scheme Policy .......................................................51 Community Impact Assessment Planning Scheme Policy .......................................................55 Compensatory Earthworks Planning Scheme Policy ................................................................. -
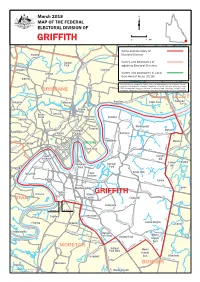
GRIFFITHE O R Kedron D W E K K Al Broo Schulz Can Name and Boundary of N E Stafford Ro Electoral Divisione Ed G
Chermside West N Virginia Chermside A T O Mcdowall H M AMIL O TO W M N Banyo y E ROAD B C R D U a a O N n A R DE OA L Nne w D r d MarchR 2018O y OAD o R R ODE lo ROA F D E MAP OF THE FEDERAL T A Creek IVE G Northgate DR Wavell Heights D N ELECTORAL DIVISION OF N O T Stafford Heights A R E S O R A O D M R Brook E ROAD T Brisbane Airport S 0 1 km B n GRIFFITHE o r Kedron d W e K k al Broo Schulz Can Name and boundary of n E Stafford ro Electoral DivisionE ed G K D U L N O M Gordon Names and boundaries of A L N E Park N adjoining Electoral Divisions D N R U A Enoggera T Clayfield Hendra Grange Names and boundaries of Local DRIVE K Government Areas (2016) N Alderley I L This map has been compiled by Spatial Vision from data supplied by the Australian Electoral Commission, Queensland Government Department of Natural Resources, Mines and Energy, T R Albion PSMAAscot Australia and Geoscience Australia. The source of data, other than Electoral Division O boundaries, is data from PSMA Australia, Geoscience Australia and the State of Queensland. BRISBANE P R I A DR TH SMI LILLEY D ek R FO G En re Hamilton GS Pinkenba Ashgrove og Windsor C KIN Eagle Farm A ger T a E W A Bowen Y Hills B risb Kelvin Herston ane Newstead r Grove Bulimba ive S R Red Hill S CLEM7 LYTT M PA ON Y W B Y Y Morningside IT NER C IN Teneriffe RD Fortitude D Murarrie Paddington R RD Y Spring Balmoral LYT N A Valley TO W Hill RD RD Hawthorne BRISBANE Murarrie Milton E N K RD Y R G C E ARRIE E A O N M R G I U R U E New Farm H N RD L D N M Brisbane T I Y C R W City W A WYNNUM RI -
Pdf, 511.35 KB
Heritage Information Please contact us for more information about this place: [email protected] -OR- phone 07 3403 8888 St Helens Key details Also known as Rosefarm Addresses At 13 Ethel Street, Camp Hill, Queensland 4152 Type of place House Period Victorian 1860-1890 Style Queenslander Lot plan L4_RP81665 Date of Information — May 2003 Page 1 Key dates Local Heritage Place Since — 1 January 2004 Date of Information — May 2003 Construction Roof: Corrugated iron; Walls: Timber People/associations John Smith Bennett (Occupant) Criterion for listing (A) Historical; (B) Rarity; (H) Historical association This high-set timber residence was constructed in 1887 for government surveyor and local dignitary John Smith Bennett and his family. In 1916, Bennett was elected member of the Coorparoo Shire Council and in 1919 was elected Mayor. He was also a prominent member of the Methodist community and was involved with the establishment of a local Methodist church group. In 1918, Bennett subdivided his landholdings around the property into a small estate and named the surrounding streets, including Ethel Street, after members of his family, further demonstrating their connection to the Camp Hill area. This house is one of a small number of surviving nineteenth century residences in Camp Hill. History The site containing this residence was originally part of a purchase of crown land made by Peter Schick and Peter Klupfel on 21 May 1859. The two partners, who were residents of Brisbane Town, paid ?64.15.0 for 64 acres and 3 roods of uncleared land described as Portion 122 in the Parish of Bulimba. -

Inner-Urban Sustainability: a Case Study of the South Brisbane Peninsula
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Queensland University of Technology ePrints Archive INNER-URBAN SUSTAINABILITY: A CASE STUDY OF THE SOUTH BRISBANE PENINSULA 1 D.J. O’Hare Abstract The combination of elements and conditions in inner-urban areas may be argued to constitute established patterns of urban sustainability. This paper develops the argument through a case study of the South Brisbane peninsula, one of Queensland’s oldest and densest inner-urban areas. For the purposes of the paper, urban sustainability is defined in the context of urban design and development. This definition highlights the interrelationship between urban form and structure, and the social and economic life of the city. The paper argues that South Brisbane demonstrates significant characteristics of ‘triple bottom line’ environmental, social and economic sustainability in a subtropical inner-urban context. The main data source is a major study of Queensland’s cultural landscapes, supplemented by analysis and interpretation of current local government planning and community advocacy for sustainability. The area is strongly supportive of sustainability in terms of residential densities; mixed land uses; coherent urban structure, with strong local centres serviceable by public transport; mixed building types and ages; diversity in culture and socioeconomic status of the population; and social capital in the form of engaged and creative communities. The management of such diverse and dynamic inner-urban areas raises challenges for sustainable urban design and development practice. Keywords: West End, Brisbane, social capital, urban design, urban sustainability. 1. Introduction South Brisbane ‘peninsula’ is bounded by the Brisbane River immediately south of the Brisbane city centre. -

Appendix I. Socio-Economic Assessment
BaT project Appendix I Socio-economic assessment Contents Appendix I. Socio-economic assessment ........................................................................................... 1 I.1 Evaluation of significance ................................................................................................ 1 I.1.1 Consequence................................................................................................................... 1 I.1.2 Likelihood ........................................................................................................................ 2 I.2 Population and demographic characteristics ................................................................... 3 Page i Appendix I. Socio-economic assessment I.1 Evaluation of significance The evaluation of significance used for this assessment considered the nature of the impact as well as the level of significance. The significance of the impact was determined by consideration of the: • consequence of the impact, based on the extent, duration and severity of the impact • likelihood, or probability of the impact occurring. The nature of the impacts may be: • positive – impacts have a positive or uplifting effect on the project-affected community and stakeholders and/or the quality of life of affected individuals, households or the community is improved • negative – impacts have a negative or oppressive effect on the project-affected community and stakeholders, and/or the quality of life of affected individuals, households or the community is diminished