Nyquist Interpolation Improves Neuron Yield in Multiunit Recordings Timothy J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Designing Commutative Cascades of Multidimensional Upsamplers And
IEEE SIGNAL PROCESSING LETTERS: SPL.SP.4.1 THEORY, ALGORITHMS, AND SYSTEMS 0 Designing Commutative Cascades of Multidimensional Upsamplers and Downsamplers Brian L. Evans, Member, IEEE Abstract In multiple dimensions, the cascade of an upsampler by L and a downsampler by L commutes if and only if the integer matrices L and M are right coprime and LM = ML. This pap er presents algorithms to design L and M that yield commutative upsampler/dowsampler cascades. We prove that commutativity is p ossible if the 1 Jordan canonical form of the rational resampling matrix R = LM is equivalent to the Smith-McMillan form of R. A necessary condition for this equivalence is that R has an eigendecomp osition and the eigenvalues are rational. B. L. Evans is with the Department of Electrical and Computer Engineering, The UniversityofTexas at Austin, Austin, TX 78712-1084, USA. E-mail: [email protected], Web: http://www.ece.utexas.edu/~b evans, Phone: 512 232-1457, Fax: 512 471-5907. This work was sp onsored in part by NSF CAREER Award under Grant MIP-9702707. July 31, 1997 DRAFT IEEE SIGNAL PROCESSING LETTERS: SPL.SP.4.1 THEORY, ALGORITHMS, AND SYSTEMS 1 I. Introduction 1 Resampling systems scale the sampling rate by a rational factor R = L=M = LM , or 1 equivalently decimate by H = M=L = L M [1], by essentially upsampling by L, ltering, and downsampling by M . In converting compact disc data sampled at 44.1 kHz to digital audio tap e 48000 Hz 160 data sampled at 48 kHz, R = = . Because we can always factor R into coprime 44100 Hz 147 integers L and M , we can always commute the upsampler and downsampler which leads to ecient p olyphase structures of the resampling system. -

Discrete - Time Signals and Systems
Discrete - Time Signals and Systems Sampling – II Sampling theorem & Reconstruction Yogananda Isukapalli Sampling at diffe- -rent rates From these figures, it can be concluded that it is very important to sample the signal adequately to avoid problems in reconstruction, which leads us to Shannon’s sampling theorem 2 Fig:7.1 Claude Shannon: The man who started the digital revolution Shannon arrived at the revolutionary idea of digital representation by sampling the information source at an appropriate rate, and converting the samples to a bit stream Before Shannon, it was commonly believed that the only way of achieving arbitrarily small probability of error in a communication channel was to 1916-2001 reduce the transmission rate to zero. All this changed in 1948 with the publication of “A Mathematical Theory of Communication”—Shannon’s landmark work Shannon’s Sampling theorem A continuous signal xt( ) with frequencies no higher than fmax can be reconstructed exactly from its samples xn[ ]= xn [Ts ], if the samples are taken at a rate ffs ³ 2,max where fTss= 1 This simple theorem is one of the theoretical Pillars of digital communications, control and signal processing Shannon’s Sampling theorem, • States that reconstruction from the samples is possible, but it doesn’t specify any algorithm for reconstruction • It gives a minimum sampling rate that is dependent only on the frequency content of the continuous signal x(t) • The minimum sampling rate of 2fmax is called the “Nyquist rate” Example1: Sampling theorem-Nyquist rate x( t )= 2cos(20p t ), find the Nyquist frequency ? xt( )= 2cos(2p (10) t ) The only frequency in the continuous- time signal is 10 Hz \ fHzmax =10 Nyquist sampling rate Sampling rate, ffsnyq ==2max 20 Hz Continuous-time sinusoid of frequency 10Hz Fig:7.2 Sampled at Nyquist rate, so, the theorem states that 2 samples are enough per period. -

The Nyquist Sampling Rate for Spiraling Curves 11
THE NYQUIST SAMPLING RATE FOR SPIRALING CURVES PHILIPPE JAMING, FELIPE NEGREIRA & JOSE´ LUIS ROMERO Abstract. We consider the problem of reconstructing a compactly supported function from samples of its Fourier transform taken along a spiral. We determine the Nyquist sampling rate in terms of the density of the spiral and show that, below this rate, spirals suffer from an approximate form of aliasing. This sets a limit to the amount of under- sampling that compressible signals admit when sampled along spirals. More precisely, we derive a lower bound on the condition number for the reconstruction of functions of bounded variation, and for functions that are sparse in the Haar wavelet basis. 1. Introduction 1.1. The mobile sampling problem. In this article, we consider the reconstruction of a compactly supported function from samples of its Fourier transform taken along certain curves, that we call spiraling. This problem is relevant, for example, in magnetic resonance imaging (MRI), where the anatomy and physiology of a person are captured by moving sensors. The Fourier sampling problem is equivalent to the sampling problem for bandlimited functions - that is, functions whose Fourier transform are supported on a given compact set. The most classical setting concerns functions of one real variable with Fourier transform supported on the unit interval [ 1/2, 1/2], and sampled on a grid ηZ, with η > 0. The sampling rate η determines whether− every bandlimited function can be reconstructed from its samples: reconstruction fails if η > 1 and succeeds if η 6 1 [42]. The transition value η = 1 is known as the Nyquist sampling rate, and it is the benchmark for all sampling schemes: modern sampling strategies that exploit the particular structure of a certain class of signals are praised because they achieve sub-Nyquist sampling rates. -

2.161 Signal Processing: Continuous and Discrete Fall 2008
MIT OpenCourseWare http://ocw.mit.edu 2.161 Signal Processing: Continuous and Discrete Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING 2.161 Signal Processing - Continuous and Discrete 1 Sampling and the Discrete Fourier Transform 1 Sampling Consider a continuous function f(t) that is limited in extent, T1 · t < T2. In order to process this function in the computer it must be sampled and represented by a ¯nite set of numbers. The most common sampling scheme is to use a ¯xed sampling interval ¢T and to form a sequence of length N: ffng (n = 0 : : : N ¡ 1), where fn = f(T1 + n¢T ): In subsequent processing the function f(t) is represented by the ¯nite sequence ffng and the sampling interval ¢T . In practice, sampling occurs in the time domain by the use of an analog-digital (A/D) converter. The mathematical operation of sampling (not to be confused with the physics of sampling) is most commonly described as a multiplicative operation, in which f(t) is multiplied by a Dirac comb sampling function s(t; ¢T ), consisting of a set of delayed Dirac delta functions: X1 s(t; ¢T ) = ±(t ¡ n¢T ): (1) n=¡1 ? We denote the sampled waveform f (t) as X1 ? f (t) = s(t; ¢T )f(t) = f(t)±(t ¡ n¢T ) (2) n=¡1 ? as shown in Fig. 1. Note that f (t) is a set of delayed weighted delta functions, and that the waveform must be interpreted in the distribution sense by the strength (or area) of each component impulse. -

Signal Sampling
FYS3240 PC-based instrumentation and microcontrollers Signal sampling Spring 2017 – Lecture #5 Bekkeng, 30.01.2017 Content – Aliasing – Sampling – Analog to Digital Conversion (ADC) – Filtering – Oversampling – Triggering Analog Signal Information Three types of information: • Level • Shape • Frequency Sampling Considerations – An analog signal is continuous – A sampled signal is a series of discrete samples acquired at a specified sampling rate – The faster we sample the more our sampled signal will look like our actual signal Actual Signal – If not sampled fast enough a problem known as aliasing will occur Sampled Signal Aliasing Adequately Sampled SignalSignal Aliased Signal Bandwidth of a filter • The bandwidth B of a filter is defined to be between the -3 dB points Sampling & Nyquist’s Theorem • Nyquist’s sampling theorem: – The sample frequency should be at least twice the highest frequency contained in the signal Δf • Or, more correctly: The sample frequency fs should be at least twice the bandwidth Δf of your signal 0 f • In mathematical terms: fs ≥ 2 *Δf, where Δf = fhigh – flow • However, to accurately represent the shape of the ECG signal signal, or to determine peak maximum and peak locations, a higher sampling rate is required – Typically a sample rate of 10 times the bandwidth of the signal is required. Illustration from wikipedia Sampling Example Aliased Signal 100Hz Sine Wave Sampled at 100Hz Adequately Sampled for Frequency Only (Same # of cycles) 100Hz Sine Wave Sampled at 200Hz Adequately Sampled for Frequency and Shape 100Hz Sine Wave Sampled at 1kHz Hardware Filtering • Filtering – To remove unwanted signals from the signal that you are trying to measure • Analog anti-aliasing low-pass filtering before the A/D converter – To remove all signal frequencies that are higher than the input bandwidth of the device. -

Deep Image Prior for Undersampling High-Speed Photoacoustic Microscopy
Photoacoustics 22 (2021) 100266 Contents lists available at ScienceDirect Photoacoustics journal homepage: www.elsevier.com/locate/pacs Deep image prior for undersampling high-speed photoacoustic microscopy Tri Vu a,*, Anthony DiSpirito III a, Daiwei Li a, Zixuan Wang c, Xiaoyi Zhu a, Maomao Chen a, Laiming Jiang d, Dong Zhang b, Jianwen Luo b, Yu Shrike Zhang c, Qifa Zhou d, Roarke Horstmeyer e, Junjie Yao a a Photoacoustic Imaging Lab, Duke University, Durham, NC, 27708, USA b Department of Biomedical Engineering, Tsinghua University, Beijing, 100084, China c Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA, 02139, USA d Department of Biomedical Engineering and USC Roski Eye Institute, University of Southern California, Los Angeles, CA, 90089, USA e Computational Optics Lab, Duke University, Durham, NC, 27708, USA ARTICLE INFO ABSTRACT Keywords: Photoacoustic microscopy (PAM) is an emerging imaging method combining light and sound. However, limited Convolutional neural network by the laser’s repetition rate, state-of-the-art high-speed PAM technology often sacrificesspatial sampling density Deep image prior (i.e., undersampling) for increased imaging speed over a large field-of-view. Deep learning (DL) methods have Deep learning recently been used to improve sparsely sampled PAM images; however, these methods often require time- High-speed imaging consuming pre-training and large training dataset with ground truth. Here, we propose the use of deep image Photoacoustic microscopy Raster scanning prior (DIP) to improve the image quality of undersampled PAM images. Unlike other DL approaches, DIP requires Undersampling neither pre-training nor fully-sampled ground truth, enabling its flexible and fast implementation on various imaging targets. -

ELEG 5173L Digital Signal Processing Ch. 3 Discrete-Time Fourier Transform
Department of Electrical Engineering University of Arkansas ELEG 5173L Digital Signal Processing Ch. 3 Discrete-Time Fourier Transform Dr. Jingxian Wu [email protected] 2 OUTLINE • The Discrete-Time Fourier Transform (DTFT) • Properties • DTFT of Sampled Signals • Upsampling and downsampling 3 DTFT • Discrete-time Fourier Transform (DTFT) X () x(n)e jn n – (radians): digital frequency • Review: Z-transform: X (z) x(n)zn n0 j X () X (z) | j – Replace z with e . ze • Review: Fourier transform: X () x(t)e jt – (rads/sec): analog frequency 4 DTFT • Relationship between DTFT and Fourier Transform – Sample a continuous time signal x a ( t ) with a sampling period T xs (t) xa (t) (t nT ) xa (nT ) (t nT ) n n – The Fourier Transform of ys (t) jt jnT X s () xs (t)e dt xa (nT)e n – Define: T • : digital frequency (unit: radians) • : analog frequency (unit: radians/sec) – Let x(n) xa (nT) X () X s T 5 DTFT • Relationship between DTFT and Fourier Transform (Cont’d) – The DTFT can be considered as the scaled version of the Fourier transform of the sampled continuous-time signal jt jnT X s () xs (t)e dt xa (nT)e n x(n) x (nT) T a jn X () X s x(n)e T n 6 DTFT • Discrete Frequency – Unit: radians (the unit of continuous frequency is radians/sec) – X ( ) is a periodic function with period 2 j2 n jn j2n jn X ( 2 ) x(n)e x(n)e e x(n)e X () n n n – We only need to consider for • For Fourier transform, we need to consider 1 – f T 2 2T 1 – f T 2 2T 7 DTFT • Example: find the DTFT of the following signal – 1. -
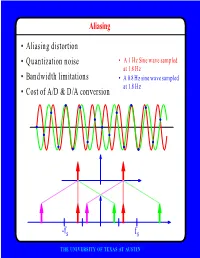
F • Aliasing Distortion • Quantization Noise • Bandwidth Limitations • Cost of A/D & D/A Conversion
Aliasing • Aliasing distortion • Quantization noise • A 1 Hz Sine wave sampled at 1.8 Hz • Bandwidth limitations • A 0.8 Hz sine wave sampled at 1.8 Hz • Cost of A/D & D/A conversion -fs fs THE UNIVERSITY OF TEXAS AT AUSTIN Advantages of Digital Systems Perfect reconstruction of a Better trade-off between signal is possible even after bandwidth and noise severe distortion immunity performance digital analog bandwidth Increase signal-to-noise ratio simply by adding more bits SNR = -7.2 + 6 dB/bit THE UNIVERSITY OF TEXAS AT AUSTIN Advantages of Digital Systems Programmability • Modifiable in the field • Implement multiple standards • Better user interfaces • Tolerance for changes in specifications • Get better use of hardware for low-speed operations • Debugging • User programmability THE UNIVERSITY OF TEXAS AT AUSTIN Disadvantages of Digital Systems Programmability • Speed is too slow for some applications • High average power and peak power consumption RISC (2 Watts) vs. DSP (50 mW) DATA PROG MEMORY MEMORY HARVARD ARCHITECTURE • Aliasing from undersampling • Clipping from quantization Q[v] v v THE UNIVERSITY OF TEXAS AT AUSTIN Analog-to-Digital Conversion 1 --- T h(t) Q[.] xt() yt() ynT() yˆ()nT Anti-Aliasing Sampler Quantizer Filter xt() y(nT) t n y(t) ^y(nT) t n THE UNIVERSITY OF TEXAS AT AUSTIN Resampling Changing the Sampling Rate • Conversion between audio formats Compact 48.0 Digital Disc ---------- Audio Tape 44.1 KHz44.1 48 KHz • Speech compression Speech 1 Speech for on DAT --- Telephone 48 KHz 6 8 KHz • Video format conversion -

Evaluating Oscilloscope Sample Rates Vs. Sampling Fidelity: How to Make the Most Accurate Digital Measurements
AC 2011-2914: EVALUATING OSCILLOSCOPE SAMPLE RATES VS. SAM- PLING FIDELITY Johnnie Lynn Hancock, Agilent Technologies About the Author Johnnie Hancock is a Product Manager at Agilent Technologies Digital Test Division. He began his career with Hewlett-Packard in 1979 as an embedded hardware designer, and holds a patent for digital oscillo- scope amplifier calibration. Johnnie is currently responsible for worldwide application support activities that promote Agilent’s digitizing oscilloscopes and he regularly speaks at technical conferences world- wide. Johnnie graduated from the University of South Florida with a degree in electrical engineering. In his spare time, he enjoys spending time with his four grandchildren and restoring his century-old Victorian home located in Colorado Springs. Contact Information: Johnnie Hancock Agilent Technologies 1900 Garden of the Gods Rd Colorado Springs, CO 80907 USA +1 (719) 590-3183 johnnie [email protected] c American Society for Engineering Education, 2011 Evaluating Oscilloscope Sample Rates vs. Sampling Fidelity: How to Make the Most Accurate Digital Measurements Introduction Digital storage oscilloscopes (DSO) are the primary tools used today by digital designers to perform signal integrity measurements such as setup/hold times, rise/fall times, and eye margin tests. High performance oscilloscopes are also widely used in university research labs to accurately characterize high-speed digital devices and systems, as well as to perform high energy physics experiments such as pulsed laser testing. In addition, general-purpose oscilloscopes are used extensively by Electrical Engineering students in their various EE analog and digital circuits lab courses. The two key banner specifications that affect an oscilloscope’s signal integrity measurement accuracy are bandwidth and sample rate. -

Enhancing ADC Resolution by Oversampling
AVR121: Enhancing ADC resolution by oversampling 8-bit Features Microcontrollers • Increasing the resolution by oversampling • Averaging and decimation • Noise reduction by averaging samples Application Note 1 Introduction Atmel’s AVR controller offers an Analog to Digital Converter with 10-bit resolution. In most cases 10-bit resolution is sufficient, but in some cases higher accuracy is desired. Special signal processing techniques can be used to improve the resolution of the measurement. By using a method called ‘Oversampling and Decimation’ higher resolution might be achieved, without using an external ADC. This Application Note explains the method, and which conditions need to be fulfilled to make this method work properly. Figure 1-1. Enhancing the resolution. A/D A/D A/D 10-bit 11-bit 12-bit t t t Rev. 8003A-AVR-09/05 2 Theory of operation Before reading the rest of this Application Note, the reader is encouraged to read Application Note AVR120 - ‘Calibration of the ADC’, and the ADC section in the AVR datasheet. The following examples and numbers are calculated for Single Ended Input in a Free Running Mode. ADC Noise Reduction Mode is not used. This method is also valid in the other modes, though the numbers in the following examples will be different. The ADCs reference voltage and the ADCs resolution define the ADC step size. The ADC’s reference voltage, VREF, may be selected to AVCC, an internal 2.56V / 1.1V reference, or a reference voltage at the AREF pin. A lower VREF provides a higher voltage precision but minimizes the dynamic range of the input signal. -

Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform Foundations of Digital Signal Processing
Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform Foundations of Digital Signal Processing Outline • Review of Sampling • The Nyquist-Shannon Sampling Theorem • Continuous-time Reconstruction / Interpolation • Aliasing and anti-Aliasing • Deriving Transforms from the Fourier Transform • Discrete-time Fourier Transform, Fourier Series, Discrete-time Fourier Series • The Discrete Fourier Transform Foundations of Digital Signal Processing Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform 1 News Homework #5 . Due this week . Submit via canvas Coding Problem #4 . Due this week . Submit via canvas Foundations of Digital Signal Processing Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform 2 Exam 1 Grades The class did exceedingly well . Mean: 89.3 . Median: 91.5 Foundations of Digital Signal Processing Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform 3 Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform Foundations of Digital Signal Processing Outline • Review of Sampling • The Nyquist-Shannon Sampling Theorem • Continuous-time Reconstruction / Interpolation • Aliasing and anti-Aliasing • Deriving Transforms from the Fourier Transform • Discrete-time Fourier Transform, Fourier Series, Discrete-time Fourier Series • The Discrete Fourier Transform Foundations of Digital Signal Processing Lecture 12: Sampling, Aliasing, and the Discrete Fourier Transform 4 Sampling Discrete-Time Fourier Transform Foundations of Digital Signal Processing Lecture 12: Sampling, Aliasing, -

Adaptive Sub-Nyquist Spectrum Sensing for Ultra-Wideband Communication Systems †
S S symmetry Article Adaptive Sub-Nyquist Spectrum Sensing for Ultra-Wideband Communication Systems † Yong Lu , Shaohe Lv and Xiaodong Wang * Science and Technology on Parallel and Distributed Processing Laboratory, National University of Defense Technology, Changsha 410073, China; [email protected] (Y.L.); [email protected] (S.L.) * Correspondence: [email protected] † This paper is an extended version of our paper published in 2014 IEEE 17th International Conference on Computational Science and Engineering (CSE), Chengdu, China, 19–21 December 2014. Received: 14 January 2019; Accepted: 3 March 2019; Published: 7 March 2019 Abstract: With the ever-increasing demand for high-speed wireless data transmission, ultra-wideband spectrum sensing is critical to support the cognitive communication over an ultra-wide frequency band for ultra-wideband communication systems. However, it is challenging for the analog-to-digital converter design to fulfill the Nyquist rate for an ultra-wideband frequency band. Therefore, we explore the spectrum sensing mechanism based on the sub-Nyquist sampling and conduct extensive experiments to investigate the influence of sampling rate, bandwidth resolution and the signal-to-noise ratio on the accuracy of sub-Nyquist spectrum sensing. Afterward, an adaptive policy is proposed to determine the optimal sampling rate, and bandwidth resolution when the spectrum occupation or the strength of the existing signals is changed. The performance of the policy is verified by simulations. Keywords: ultra-wideband spectrum sensing; energy detection; cognitive radio; sub-Nyquist sensing; Chinese remainder theorem 1. Introduction After decades of rapid development, wireless technologies have been intensively applied to almost every field from daily communications to mobile medical and battlefield communications.