Native Pine Woodlands (Uk Bap Priority Habitat)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Estimating Numbers of Embryonic Lethals in Conifers
Heredity 69(1992)308—314 Received 26 November 1991 OThe Genetical Society of Great Britain Estimating numbers of embryonic lethals in conifers OUTI SAVOLAINEN, KATRI KARKKAINEN & HELMI KUITTINEN* Department of Genetics, University of Oulu, Oulu, Fin/and and *Department of Genetics, University of Helsinki, He/sink,, Fin/and Conifershave recessive lethal genes that eliminate most selfed embryos during seed development. It has been estimated that Scots pine has, on average, nine recessive lethals which act during seed development. Such high numbers are not consistent with the level of outcrossing, about 0.9—0.95, which has been observed in natural populations. Correcting for environmental mortality or using partial selfings provides significantly lower estimates of lethals. A similar discordance with numbers of lethals and observed outcrossing rates is true for other species. Keywords:embryoniclethals, inbreeding depression, outcrossing, Pinus sylvestris, Picea omorika. Introduction Reproduction system of conifers Conifershave no self-incompatibility mechanisms but Theproportion of self-pollination in conifers is early-acting inbreeding depression eliminates selfed variable. Sarvas (1962) suggested an average of 26 per embryos before seed maturation (Sarvas, 1962; cent for Pinus sylvestris, while Koski (1970) estimated Hagman & Mikkola, 1963). A genetic model for this values of self-fertilization around 10 per cent. The inbreeding depression has been developed by Koski genera Pinus and Picea have polyzygotic poly- (1971) and Bramlett & Popham (1971). Koski (1971, embryony, i.e. the ovules contain several archegonia. In 1973) has estimated that Pinus sylvestris and Picea Pinus sylvestris, the most common number of arche- abies have on average nine and 10 recessive lethals, gonia is two but it can range from one to five. -

Diptera) of North-Eastern North America
Biodiversity Data Journal 7: e36673 doi: 10.3897/BDJ.7.e36673 Taxonomic Paper New Syrphidae (Diptera) of North-eastern North America Jeffrey H. Skevington‡,§, Andrew D. Young|, Michelle M. Locke‡, Kevin M. Moran‡,§ ‡ AAFC, Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Canada § Carleton University, Ottawa, Canada | California Department of Food and Agriculture, Sacramento, United States of America Corresponding author: Jeffrey H. Skevington ([email protected]) Academic editor: Torsten Dikow Received: 31 May 2019 | Accepted: 09 Aug 2019 | Published: 03 Sep 2019 Citation: Skevington JH, Young AD, Locke MM, Moran KM (2019) New Syrphidae (Diptera) of North-eastern North America. Biodiversity Data Journal 7: e36673. https://doi.org/10.3897/BDJ.7.e36673 ZooBank: urn:lsid:zoobank.org:pub:823430AD-B648-414F-A8B2-4F1E5F1A086A Abstract Background This paper describes 11 of 18 new species recognised in the recent book, "Field Guide to the Flower Flies of Northeastern North America". Four species are omitted as they need to be described in the context of a revision (three Cheilosia and a Palpada species) and three other species (one Neoascia and two Xylota) will be described by F. Christian Thompson in a planned publication. Six of the new species have been recognised for decades and were treated by J. Richard Vockeroth in unpublished notes or by Thompson in his unpublished but widely distributed "A conspectus of the flower flies (Diptera: Syrphidae) of the Nearctic Region". Five of the 11 species were discovered during the preparation of the Field Guide. Eight of the 11 have DNA barcodes available that support the morphology. New information New species treated in this paper include: Anasimyia diffusa Locke, Skevington and Vockeroth (Smooth-legged Swamp Fly), Anasimyia matutina Locke, Skevington and This is an open access article distributed under the terms of the CC0 Public Domain Dedication. -

SILVER BIRCH (BETULA PENDULA) at 200-230°C Raimo Alkn Risto Kotilainen
THERMAL BEHAVIOR OF SCOTS PINE (PINUS SYLVESTRIS) AND SILVER BIRCH (BETULA PENDULA) AT 200-230°C Postgraduate Student Raimo Alkn Professor and Risto Kotilainen Postgraduate Student Department of Chemistry, Laboratory of Applied Chemistry University of Jyvaskyla, PO. Box 35, FIN-40351 Jyvaskyla, Finland (Received April 1998) ABSTRACT Scots pine (Pinus sylvestris) and silver birch (Betuln pendula) were heated for 4-8 h in a steam atmosphere at low temperatures (200-230°C). The birch feedstock decomposed slightly more exten- sively (6.4-10.2 and 13515.2% of the initial DS at 200°C and 225"C, respectively) than the pine feedstock (5.7-7.0 and 11.1-15.2% at 205°C and 230°C. respectively). The results indicated that the differences in mass loss between these feedstocks were due to mainly the fact that carbohydrates (cellulose and hemicelluloses) were more amenable to various degradation reactions than lignin in intact wood. The degradation reactions were also monitored in both cases by determining changes in the elemental composition of the heat-treated products. Keywords: Heat treatment, carbohydrates, lignin, extractives, Pinus sylvestris, Betula pendula. INTRODUCTION content of hemicelluloses (25-30% of DS) In our previous experiments (AlCn et al. than hardwood (lignin and hemicelluloses are 2000), the thermal degradation of the structur- usually in the range 20-25 and 30-35% of al constituents (lignin and polysaccharides, DS, respectively). On the basis of these facts, i.e., cellulose and hemicelluloses) of Norway the thermal behavior of softwood and hard- spruce (Picea abies) was established under wood can be expected to be different, even at conditions (temperature range 180-225"C, low temperatures. -
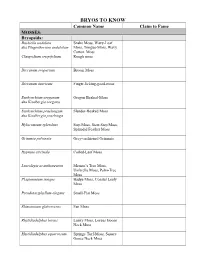
Bryo's to Know Table
BRYOS TO KNOW Common Name Claim to Fame MOSSES: Bryopsida: Buckiella undulata Snake Moss, Wavy-Leaf aka Plagiothecium undulatum Moss, Tongue-Moss, Wavy Cotton, Moss Claopodium crispifolium Rough moss Dicranum scoparium Broom Moss Dicranum tauricum Finger-licking-good-moss Eurhynchium oreganum Oregon Beaked-Moss aka Kindbergia oregana Eurhynchium praelongum Slender-Beaked Moss aka Kindbergia praelonga Hylocomium splendens Step Moss, Stair-Step Moss, Splendid Feather Moss Grimmia pulvinata Grey-cushioned Grimmia Hypnum circinale Coiled-Leaf Moss Leucolepis acanthoneuron Menzie’s Tree Moss, Umbrella Moss, Palm-Tree Moss Plagiomnium insigne Badge Moss, Coastal Leafy Moss Pseudotaxiphyllum elegans Small-Flat Moss Rhizomnium glabrescens Fan Moss Rhytidiadelphus loreus Lanky Moss, Loreus Goose Neck Moss Rhytidiadelphus squarrosum Springy Turf-Moss, Square Goose Neck Moss Rhytidiadelphus triquetrus Electrified Cat-Tail Moss, Goose Necked Moss Rhytidiopsus robusta Robust mountain moss Schistostega pennata Goblin’s Gold, Luminous Moss Polytrichopsida: Atrichum Atrichum Moss , Crane’s Bill Moss (for Atrichum selwynii) Pogonatum contortum Contorted Pogonatum Moss Polytrichum commune Common Hair Cap Moss Polytrichum piliferum Bristly Haircap Moss Andreaeopsida Andreaea nivalis Granite moss, Lantern moss, Snow Rock Moss Sphagnopsida: Sphagnum capillifolium Red Bog Moss, Small Red Peat Moss Sphagnum papillosum Fat Bog Moss, Papillose sphagnum Sphagnum squarrosum Shaggy Sphagnum, Spread- Leaved Peat Moss Takakiopsida: Takakia lepidoziooides Impossible -

Environmental
STRATEGY Cairngorms National Park Partnership Plan 2017-2022 Strategic Environmental Assessment Scoping Report Appendix 2: Environmental Baseline Topic 6: Biodiversity, Fauna and Flora November 2015 [NPPP SEA SCOPING REPORT] November 2015 Topic 6: Biodiversity, Fauna and Flora Protected Areas National Nature Reserves “Biodiversity – the variety of Life on Earth – makes our planet habitable and beautiful. Protected areas represent the very best of NNRs are statutory nature reserves We depend on it for food, energy, raw Scotland's landscapes, plants and animals, designed under Part III of the National materials, air and water that make life rocks, fossils and landforms. Their Parks and Access to the Countryside Act possible and drive our economy. We look to protection and management will help to 1949. Most reserves have habitats and the natural environment for equally ensure that they remain in good health for species that are nationally or internationally important things like aesthetic pleasure, all to enjoy, both now and for future important so the wildlife is managed very artistic inspiration and recreation.” generations. carefully. However, people are also encouraged to enjoy NNRs too and so European Commission Natura 2000. The Cairngorms National Park is home to a most have some form of visitor facilities number of areas designated to meet the that are designed to ensure recreational needs of international directives and The Cairngorms National Park is a haven activities are not pursued without heed for treaties, national legislation and policies as for nature and wildlife and is of great the wildlife and habitat that exists there. well as more local needs and interests. -

Coptis Trifolia Conservation Assessment
CONSERVATION ASSESSMENT for Coptis trifolia (L.) Salisb. Originally issued as Management Recommendations December 1998 Marty Stein Reconfigured-January 2005 Tracy L. Fuentes USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington CONSERVATION ASSESSMENT FOR COPTIS TRIFOLIA Table of Contents Page List of Tables ................................................................................................................................. 2 List of Figures ................................................................................................................................ 2 Summary........................................................................................................................................ 4 I. NATURAL HISTORY............................................................................................................. 6 A. Taxonomy and Nomenclature.......................................................................................... 6 B. Species Description ........................................................................................................... 6 1. Morphology ................................................................................................................... 6 2. Reproductive Biology.................................................................................................... 7 3. Ecological Roles ............................................................................................................. 7 C. Range and Sites -

G. Gulden & E.W. Hanssen Distribution and Ecology of Stipitate Hydnaceous Fungi in Norway, with Special Reference to The
DOI: 10.2478/som-1992-0001 sommerfeltia 13 G. Gulden & E.W. Hanssen Distribution and ecology of stipitate hydnaceous fungi in Norway, with special reference to the question of decline 1992 sommerfeltia~ J is owned and edited by the Botanical Garden and Museum, University of Oslo. SOMMERFELTIA is named in honour of the eminent Norwegian botanist and clergyman S0ren Christian Sommerfelt (1794-1838). The generic name Sommerfeltia has been used in (1) the lichens by Florke 1827, now Solorina, (2) Fabaceae by Schumacher 1827, now Drepanocarpus, and (3) Asteraceae by Lessing 1832, nom. cons. SOMMERFELTIA is a series of monographs in plant taxonomy, phytogeo graphy, phytosociology, plant ecology, plant morphology, and evolutionary botany. Most papers are by Norwegian authors. Authors not on the staff of the Botanical Garden and Museum in Oslo pay a page charge of NOK 30.00. SOMMERFEL TIA appears at irregular intervals, normally one article per volume. Editor: Rune Halvorsen 0kland. Editorial Board: Scientific staff of the Botanical Garden and Museum. Address: SOMMERFELTIA, Botanical Garden and Museum, University of Oslo, Trondheimsveien 23B, N-0562 Oslo 5, Norway. Order: On a standing order (payment on receipt of each volume) SOMMER FELTIA is supplied at 30 % discount. Separate volumes are supplied at the prices indicated on back cover. sommerfeltia 13 G. Gulden & E.W. Hanssen Distribution and ecology of stipitate hydnaceous fungi in Norway, with special reference to the question of decline 1992 ISBN 82-7420-014-4 ISSN 0800-6865 Gulden, G. and Hanssen, E.W. 1992. Distribution and ecology of stipitate hydnaceous fungi in Norway, with special reference to the question of decline. -

Blera Eoa (Stackelberg, 1928), En Ny Stubb-Blomfluga För Europa (Diptera, Syrphidae)
Natur i Norr, Umeå Årgång 20 (2001), häfte 2:91-96 Blera eoa (Stackelberg, 1928), en ny stubb-blomfluga för Europa (Diptera, Syrphidae) ROGER B. PETTERSSON & HANS D. BARTSCH Summary är sedan tidigare endast känd från Sibirien A single female of the hoverfly Blera eoa och Ryska fjärran östern (Barkalov & (Stackelberg, 1928) was collected in the Mutin 1991ab). forest reserve of Paskatieva, Norrbotten Den aktuella honan liknar stubb- province, North Sweden. The fly was blomflugan Blera fallax (Linnaeus, 1758) found in a window trap placed on a pine (Fig.2). B. fallax har dock en glänsande trunk by R. Pettersson, during the samp- svart kropp med bakre delen av abdomen ling period of 2.VI-7.VII. 2000. The orangefärgad. Hos hanen upptar det species has not been reported from Europe orangefärgade området halva abdomen, before, and it is uncertain if it is a random hos honan dock enbart själva spetsen (Fig. record or if B. eoa has any reproduction 2). Behåringen på rygg och bakkropp är in this area outside its known range of gråblek så när som ett tvärband med svarta distribution. The species has an entirely hår över ryggen och orangegula hår på black abdomen and differs from B. fallax bakkroppens orangefärgade partier. as described by Barkalov & Mutin (1991 Honan från Paskatieva (Fig. 3-4) har ab). helsvart kropp med enbart gyllengul behåring på ryggen och i huvudsak svart Inledning behåring på bakkroppen. Den ryska invasionen fortsätter. Då och Ett intensivt detektivarbete startade när då har arter kända från Ryssland hitom Hans fick exemplaret av Roger i samband eller bortom Ural påträffats i Sverige. -

Examensarbete I Ämnet Biologi
Examensarbete 2012:10 i ämnet biologi Comparison of bird communities in stands of introduced lodgepole pine and native Scots pine in Sweden Arvid Alm Sveriges lantbruksuniversitet Fakulteten för skogsvetenskap Institutionen för vilt, fisk och miljö Examensarbete i biologi, 15 hp, G2E Umeå 2012 Examensarbete 2012:10 i ämnet biologi Comparison of bird communities in stands of introduced lodgepole pine and native Scots pine in Sweden Jämförelse mellan fågelsamhällen i bestånd av introducerad contortatall och inhemsk tall i Sverige Arvid Alm Keywords: Boreal forest, Pinus contorta, Pinus sylvestris, bird assemblage, Muscicapa striata Handledare: Jean-Michel Roberge & Adriaan de Jong 15 hp, G2E Examinator: Lars Edenius Kurskod EX0571 SLU, Sveriges lantbruksuniversitet Swedish University of Agricultural Sciences Fakulteten för skogsvetenskap Faculty of Forestry Institutionen för vilt, fisk och miljö Dept. of Wildlife, Fish, and Environmental Studies Umeå 2012 Abstract The introduced lodgepole pine (Pinus contorta) occupies more than 650 000 hectares in Sweden. There are some differences between lodgepole pine and Scots pine (Pinus sylvestris) forests which could affect bird assemblages, for example differences in canopy density and ground vegetation. Birds were surveyed in 14 localities in northern Sweden, each characterized by one middle-aged stand of lodgepole pine next to a stand of Scots pine. The two paired stands in each locality were planted by the forestry company SCA at the same time and in similar environment to evaluate the potential of lodgepole pine in Sweden. In those 14 localities, one to three point count stations were established in both the lodgepole pine and the Scots pine stand, depending on the size of the area. -

Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon
The Bryologist 99(2), pp. 226-235 Copyright © 1996 by the American Bryological and Lichenological Society, Inc. Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon BENGT GUNNAR JONSSON I Department of Forest Science, Oregon State University, Corvallis, OR 97331 Abstract. The knowledge of the distribution and habitat demands for bryophytes in the Pacific Northwest is scarce, and few published quantitative accounts of the flora are present. The present paper includes habitat description, elevational range, substrate preference, and frequency esti- mates for more than 130 riparian mosses and liverworts found in old-growth Pseudotsuga-Tsuga forests of the H. J. Andrews Experimental Forest, Oregon. The data are based on 360 samples distributed among 42 sites covering 1st to 5th order streams and 420 to 1250 m. TWINSPAN analysis resulted in 6 sample groups, representing samples from different elevations, geomorphic surfaces, and stream sizes. The most common mosses are Eurhynchium oreganum, Isothecium stoloniferum, Hypnum circinale, and Dicranum fuscescens. Among the hepatics Scapania bolan- deri, Cephalozia lunulifolia, and Porella navicularis are the most abundant species. Most species are rare at both site and sampte gtot (ever; and this is especially true for acrocarps where more than one-third of the observed species occurred in only one or two sites orland samples. Four of the occurring species (i.e., Antitrichia curtipendula, Buxbaumia piperi, Douinia ovata, and Ptili- dium californicum) are listed for special management and/or regional surveys. Bryophytes constitute an important and conspic- a wide range of different substrates, disturbance uous component of old-growth forests in the Pacific patterns, and a moist microclimate are important Northwest (Lesica et al. -

Molecular Phylogeny of Chinese Thuidiaceae with Emphasis on Thuidium and Pelekium
Molecular Phylogeny of Chinese Thuidiaceae with emphasis on Thuidium and Pelekium QI-YING, CAI1, 2, BI-CAI, GUAN2, GANG, GE2, YAN-MING, FANG 1 1 College of Biology and the Environment, Nanjing Forestry University, Nanjing 210037, China. 2 College of Life Science, Nanchang University, 330031 Nanchang, China. E-mail: [email protected] Abstract We present molecular phylogenetic investigation of Thuidiaceae, especially on Thudium and Pelekium. Three chloroplast sequences (trnL-F, rps4, and atpB-rbcL) and one nuclear sequence (ITS) were analyzed. Data partitions were analyzed separately and in combination by employing MP (maximum parsimony) and Bayesian methods. The influence of data conflict in combined analyses was further explored by two methods: the incongruence length difference (ILD) test and the partition addition bootstrap alteration approach (PABA). Based on the results, ITS 1& 2 had crucial effect in phylogenetic reconstruction in this study, and more chloroplast sequences should be combinated into the analyses since their stability for reconstructing within genus of pleurocarpous mosses. We supported that Helodiaceae including Actinothuidium, Bryochenea, and Helodium still attributed to Thuidiaceae, and the monophyletic Thuidiaceae s. lat. should also include several genera (or species) from Leskeaceae such as Haplocladium and Leskea. In the Thuidiaceae, Thuidium and Pelekium were resolved as two monophyletic groups separately. The results from molecular phylogeny were supported by the crucial morphological characters in Thuidiaceae s. lat., Thuidium and Pelekium. Key words: Thuidiaceae, Thuidium, Pelekium, molecular phylogeny, cpDNA, ITS, PABA approach Introduction Pleurocarpous mosses consist of around 5000 species that are defined by the presence of lateral perichaetia along the gametophyte stems. Monophyletic pleurocarpous mosses were resolved as three orders: Ptychomniales, Hypnales, and Hookeriales (Shaw et al. -

Lichens and Associated Fungi from Glacier Bay National Park, Alaska
The Lichenologist (2020), 52,61–181 doi:10.1017/S0024282920000079 Standard Paper Lichens and associated fungi from Glacier Bay National Park, Alaska Toby Spribille1,2,3 , Alan M. Fryday4 , Sergio Pérez-Ortega5 , Måns Svensson6, Tor Tønsberg7, Stefan Ekman6 , Håkon Holien8,9, Philipp Resl10 , Kevin Schneider11, Edith Stabentheiner2, Holger Thüs12,13 , Jan Vondrák14,15 and Lewis Sharman16 1Department of Biological Sciences, CW405, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; 2Department of Plant Sciences, Institute of Biology, University of Graz, NAWI Graz, Holteigasse 6, 8010 Graz, Austria; 3Division of Biological Sciences, University of Montana, 32 Campus Drive, Missoula, Montana 59812, USA; 4Herbarium, Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824, USA; 5Real Jardín Botánico (CSIC), Departamento de Micología, Calle Claudio Moyano 1, E-28014 Madrid, Spain; 6Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden; 7Department of Natural History, University Museum of Bergen Allégt. 41, P.O. Box 7800, N-5020 Bergen, Norway; 8Faculty of Bioscience and Aquaculture, Nord University, Box 2501, NO-7729 Steinkjer, Norway; 9NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway; 10Faculty of Biology, Department I, Systematic Botany and Mycology, University of Munich (LMU), Menzinger Straße 67, 80638 München, Germany; 11Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; 12Botany Department, State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany; 13Natural History Museum, Cromwell Road, London SW7 5BD, UK; 14Institute of Botany of the Czech Academy of Sciences, Zámek 1, 252 43 Průhonice, Czech Republic; 15Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic and 16Glacier Bay National Park & Preserve, P.O.