Cuttlefish Biology Geetha Sasikumar Sr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproductive Behavior of the Japanese Spineless Cuttlefish Sepiella Japonica
VENUS 65 (3): 221-228, 2006 Reproductive Behavior of the Japanese Spineless Cuttlefish Sepiella japonica Toshifumi Wada1*, Takeshi Takegaki1, Tohru Mori2 and Yutaka Natsukari1 1Graduate School of Science and Technology, Nagasaki University, 1-14 Bunkyo-machi, Nagasaki 852-8521, Japan 2Marine World Uminonakamichi, 18-28 Saitozaki, Higashi-ku, Fukuoka 811-0321, Japan Abstract: The reproductive behavior of the Japanese spineless cuttlefish Sepiella japonica was observed in a tank. The males competed for females before egg-laying and then formed pairs with females. The male then initiated mating by pouncing on the female head, and maintained the male superior head-to-head position during the mating. Before ejaculation, the male moved his right (non-hectocotylized) arm IV under the ventral portion of the female buccal membrane, resulting in the dropping of parts of spermatangia placed there during previous matings. After the sperm removal behavior, the male held spermatophores ejected through his funnel with the base of hectocotylized left arm IV and transferred them to the female buccal area. The spermatophore transfer occurred only once during each mating. The female laid an egg capsule at average intervals of 1.5 min and produced from 36 to more than 408 egg capsules in succession during a single egg-laying bout. Our results also suggested one female produced nearly 200 fertilized eggs without additional mating, implying that the female have potential capacity to store and use active sperm properly. The male continued to guard the spawning female after mating (range=41.8-430.1 min), and repeated matings occurred at an average interval of 70.8 min during the mate guarding. -

Cuttlefish, <I>Sepia</I>
Marine Science 2015, 5(1): 6-10 DOI: 10.5923/j.ms.20150501.02 Size at First Maturity of Cuttlefish, Sepia latimanus, from North Sulawesi Waters, Indonesia Silvester B. Pratasik1,2,*, Marsoedi3, D. Arfiati3, D. Setyohadi3 1Postgraduate student of Faculty of Fisheries and Marine Science, Brawijaya University, Malang, East Java 2Faculty of Fisheries and Marine Science, Sam Ratulangi Manado, North Sulawesi 3Faculty of Fisheries and Marine Science, Brawijaya University Malang, East Java Abstract Biological overfishing could occur from either excessive and immature individual exploitation or habitat destruction. This study was aimed to estimate size at first maturity of cuttlefish, Sepia latimanus, collected from North Sulawesi waters. All samples were measured and observed their maturity level. Based on these data, the dorsal mantle length (DML) at first maturity was assessed for minimum legal size determination. Results showed that the cuttlefish samples had maturity level range from immature to post-spawning conditions, while the size at first maturity was estimated as 16 cm DML. Maximum DML was estimated as 55.53 cm and growth coefficient as 0.248. The deviation of mean DML from maximum dorsal length was also considered to see the population condition. Keywords Cuttlefish, Sepia latimanus, Size at first maturity than that maximizing the economic rent (economic 1. Introduction overfishing) [8]. Size at first maturity (lm) or 50% of mature individuals has been taken as reference point of minimum Cuttlefish are one of the high economic value exported legal size to prevent stock depletion. It has been used by fisheries resources, and therefore, they are highly exploited many fisheries managers as management measure of fish in the world. -

Life Sciences, 2018; 6 (3):799-806 Life Sciences ISSN:2320-7817(P) | 2320-964X(O)
International Journal of Int. J. of Life Sciences, 2018; 6 (3):799-806 Life Sciences ISSN:2320-7817(p) | 2320-964X(o) International Peer Reviewed Open Access Refereed Journal Original Article Open Access Species diversity and basic biology of Cuttlefishes from Maharashtra waters, northwest coast of India Sundaram Sujit1 and Mane Sushant 2 1 Mumbai Research Centre of Central Marine Fisheries Research Institute, 2nd Floor, C.I.F.E old campus, Fisheries University road, Seven Bunglows, Versova, Mumbai - 400 061, Maharashtra, India. (Retd.) 2 Department of Zoology, Wilson College, Chowpaty, Mumbai-400 007, Maharashtra, India. Email- [email protected] Manuscript details: ABSTRACT Received :11.06.2018 Cuttlefish diversity was studied from Maharashtra waters during the period Accepted : 18.09.2018 January 2000 - December 2017. Eight species were identified and they are Published : 30.09.2018 Sepia pharaonis Ehrenberg, 1831, Sepia aculeata Orbigny, 1848, Sepia elliptica Hoyle, 1885, Sepiella inermis Orbigny, 1848, Sepia prashadi Editor: Dr. Arvind Chavhan Winckworth, 1936, Sepia (Doratosepion) kobiensis Hoyle, 1885, Sepia omani Cite this article as: Adam and Rees, 1966 and Euprymna berryi Sasaki, 1929. The estimated Sundaram Sujit and Mane Sushant annual catch of cuttlefishes by trawlers (all species combined) for the period (2018) Species diversity and basic 2000-2017 from New Ferry Wharf landing centre showed a cyclic trend and biology of Cuttlefishes from the landings ranged from 1360.4 t (2002) to a peak of 3,704.1 t (2012) and Maharashtra waters, northwest the corresponding catch rate ranged from 0.985 kg/hr (2002) to 1.599 coast of India, Int. J. -

An Eocene Orthocone from Antarctica Shows Convergent Evolution of Internally Shelled Cephalopods
RESEARCH ARTICLE An Eocene orthocone from Antarctica shows convergent evolution of internally shelled cephalopods Larisa A. Doguzhaeva1*, Stefan Bengtson1, Marcelo A. Reguero2, Thomas MoÈrs1 1 Department of Palaeobiology, Swedish Museum of Natural History, Stockholm, Sweden, 2 Division Paleontologia de Vertebrados, Museo de La Plata, Paseo del Bosque s/n, B1900FWA, La Plata, Argentina * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Background The Subclass Coleoidea (Class Cephalopoda) accommodates the diverse present-day OPEN ACCESS internally shelled cephalopod mollusks (Spirula, Sepia and octopuses, squids, Vampyro- teuthis) and also extinct internally shelled cephalopods. Recent Spirula represents a unique Citation: Doguzhaeva LA, Bengtson S, Reguero MA, MoÈrs T (2017) An Eocene orthocone from coleoid retaining shell structures, a narrow marginal siphuncle and globular protoconch that Antarctica shows convergent evolution of internally signify the ancestry of the subclass Coleoidea from the Paleozoic subclass Bactritoidea. shelled cephalopods. PLoS ONE 12(3): e0172169. This hypothesis has been recently supported by newly recorded diverse bactritoid-like doi:10.1371/journal.pone.0172169 coleoids from the Carboniferous of the USA, but prior to this study no fossil cephalopod Editor: Geerat J. Vermeij, University of California, indicative of an endochochleate branch with an origin independent from subclass Bactritoi- UNITED STATES dea has been reported. Received: October 10, 2016 Accepted: January 31, 2017 Methodology/Principal findings Published: March 1, 2017 Two orthoconic conchs were recovered from the Early Eocene of Seymour Island at the tip Copyright: © 2017 Doguzhaeva et al. This is an of the Antarctic Peninsula, Antarctica. They have loosely mineralized organic-rich chitin- open access article distributed under the terms of compatible microlaminated shell walls and broadly expanded central siphuncles. -

Along the Saudi Arabian Red Sea Coastline Thesis by Gordon Byron
Phylogenetic Diversity of Cephalopoda (Animalia:Mollusca) Along the Saudi Arabian Red Sea Coastline Thesis by Gordon Byron In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia © December, 2016 Gordon Byron All rights reserved 2 EXAMINATION COMMITTEE PAGE The thesis of Gordon Byron is approved by the examination committee. Committee Chairperson: Michael Berumen Committee Co-Chair: Christian Voolstra Committee Member: Timothy Ravasi 3 ABSTRACT Phylogenetic Diversity of Cephalopoda (Animalia:Mollusca) Along the Saudi Red Sea Coastline Gordon Byron Although the Red Sea presents a unique environment with high temperature and salinity, it remains an area that is understudied. This lack of information is reflected in many areas, one which is biodiversity. Despite increasing work on biodiversity throughout the Red Sea and an increase in Cephalopoda studies, Cephalopoda in the Red Sea remain underrepresented, which is especially pronounced in molecular analyses. Members of the class Cephalopoda are considered to be major contributors to coral reef ecosystems, serving as part of the food chain and exhibiting population increases due to targeted teleost fisheries and global climate change. In order to assess the biodiversity of Cephalopoda in the Saudi Arabian Red Sea, 87 specimens were collected from 25 reef locations between 17°N and 28°N latitude, as well as from the largest fish market in the Kingdom of Saudi Arabia. Taxonomic identification of specimens was determined using morphological comparisons with previously reported species in the Red Sea and the molecular barcoding region Cytochrome Oxidase I. 84 Red Sea sequences were compared with sequences from GenBank and analyzed using a complement of Neighbor- Joining, Maximum-Likelihood, and Bayesian inference trees. -

House Reef Project Report Katerina Bengtsson Kupcik-2009
Lembeh Resort House Reef Project July 2009 Katerina Bengtsson Kupcik fl[email protected] House reef project status report 2009 This is a write-up of observations of the house reef project based on dives conducted 21-26 July 2009. This is a comparative study to our previous work on the House reef (HSR) during November 2006- April 2008. There are several components to the house reef project, and each of these is discussed separately in the following report. Wreck In December 2007 a 15m long wooden boat was deliberately sunk on the deep flat sand of the HSR (about 24msw). Prior to this the boat was half exposed in the neighbouring village, Pintu Kota Kecil. The wreck is now falling apart. There is not much left of the side planks, these are rotting away. The bow has fallen down altogether. What is remaining is the skeleton of the boat and the rocks piled up inside the wreck, which were used to sink it initially. Schooling fish have been observed on the wreck since the first day of its sinking. Only some of the species found on the wreck have changed from April 2008, when it was last observed by us*. There are now schooling banner butterflyfish, instead of Moorish idols, and snappers instead of sweetlips, but no result can be drawn from this. Smaller gropers (cods) are still present, mostly inside the wreck. There is not much growth on the structure itself, mostly ascidians and no corals, probably because it is quite deep. As in 2008 there is still a layer of silt on the wreck, so no mentionable sediment deposition is noticeable. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Marine Biodiversity in India
MARINEMARINE BIODIVERSITYBIODIVERSITY ININ INDIAINDIA MARINE BIODIVERSITY IN INDIA Venkataraman K, Raghunathan C, Raghuraman R, Sreeraj CR Zoological Survey of India CITATION Venkataraman K, Raghunathan C, Raghuraman R, Sreeraj CR; 2012. Marine Biodiversity : 1-164 (Published by the Director, Zool. Surv. India, Kolkata) Published : May, 2012 ISBN 978-81-8171-307-0 © Govt. of India, 2012 Printing of Publication Supported by NBA Published at the Publication Division by the Director, Zoological Survey of India, M-Block, New Alipore, Kolkata-700 053 Printed at Calcutta Repro Graphics, Kolkata-700 006. ht³[eg siJ rJrJ";t Œtr"fUhK NATIONAL BIODIVERSITY AUTHORITY Cth;Govt. ofmhfUth India ztp. ctÖtf]UíK rvmwvtxe yÆgG Dr. Balakrishna Pisupati Chairman FOREWORD The marine ecosystem is home to the richest and most diverse faunal and floral communities. India has a coastline of 8,118 km, with an exclusive economic zone (EEZ) of 2.02 million sq km and a continental shelf area of 468,000 sq km, spread across 10 coastal States and seven Union Territories, including the islands of Andaman and Nicobar and Lakshadweep. Indian coastal waters are extremely diverse attributing to the geomorphologic and climatic variations along the coast. The coastal and marine habitat includes near shore, gulf waters, creeks, tidal flats, mud flats, coastal dunes, mangroves, marshes, wetlands, seaweed and seagrass beds, deltaic plains, estuaries, lagoons and coral reefs. There are four major coral reef areas in India-along the coasts of the Andaman and Nicobar group of islands, the Lakshadweep group of islands, the Gulf of Mannar and the Gulf of Kachchh . The Andaman and Nicobar group is the richest in terms of diversity. -

POPULATION DYNAMICS of the HOODED CUTTLEFISH Sepia Prashadi (WINCKWORTH, 1936) from the OMANI COASTAL WATERS of the ARABIAN SEA
7(1): 89-98 (2013) DOI: 10.3153/jfscom.2013010 Journal of FisheriesSciences.com E-ISSN 1307-234X © 2013 www.fisheriessciences.com RESEARCH ARTICLE ARAŞTIRMA MAKALESİ POPULATION DYNAMICS OF THE HOODED CUTTLEFISH Sepia prashadi (WINCKWORTH, 1936) FROM THE OMANI COASTAL WATERS OF THE ARABIAN SEA Sahar F. Mehanna∗, Dawood Al-Mamry Marine Science and Fisheries Centre, Muscat, OMAN Abstract: Basic population parameters of the hooded cuttlefish Sepia prashadi, in the Arabian Sea were described from samples collected during the demersal trawl survey of the Arabian Sea between September 2007 and August 2008. A total of 6869 S. prashadi with mantle lengths (ML) ranged from 3.4 to 21.2 cm were analyzed. Age and growth were studied using progression analysis model by applying Bhattacharya method. There were no significant differences in population parameters between sexes. The asymptotic ML was 24.13 cm, while the growth co- efficient K was 0.81/year and t0= -0.14 year. Mean total, natural and fishing mortalities were 3.66, 1.54 and 2.12 per year respectively. The exploitation ratio (E =0.58) suggests that the fishing pressure on S. prashadi in the Omani coastal waters is slightly high. Relative yield per recruit and relative biomass per recruit analysis showed that S. prashadi stock in the Arabian Sea is in its optimum situation as the current E is lower than that which gives the maximum Y’/R. For the management purpose and to reduce the risk due to the sampling bias, the current exploitation rate should be reduced by about 38% to achieve E0.5 as a target reference point and the present length at first capture should be raised to about 14 cm ML to conserve the first spawners of the stock. -

ISSN 0704-3716 Canadian Translation of Fisheries and Aquatic
ISSN 0704-3716 Canadian Translation of Fisheries and Aquatic Sciences No. 5377 Summaries of reports presented to the 4th All-Union Conference on Commercial Invertebrates Original title: Tezisy dokladov, IV Vsesoyuznaya konferentsiya po promyslovym bespozvonochnym. Chastu 1,2. 370 p. 1986. Publisher: All-Union Scientific Research Institute of Marine Fisheries and Oceanography (VNIRO). Moscow Original language: Russian Available from: Canada Institute for Scientific and Technical Information National Research Council Ottawa, Ontario, Canada KlA 0S2 1988 476 typescript pages 90 - 01551/ Secretary Secrétariat of State d'État MULTILINGUAL ERVICES DIVISION — DIVISION DES SERVICES MULTILINGUES TRANSLATION BUREAU BUREAU DES TRADUCTIONS LIBRARY IDENTIFICATION — FICHE SIGNALÉTIQUE Translated from - Traduction de Into - En Russian English Author - Auteur Title in English or French - Titre anglais ou français Summaries of reports presented to the 4th All—Union Conference on Commercial Invertebrates Title in foreign language (Transliterate foreign characters) Titre en langue étrangère (Transcrire en caractères romains) Tezisy dokladov,IV Vsesoyuznaya konferentsiya po promyslovym bespozvonochnym Reference in foreign language (Name of book or publication) in full, transliterate foreign characters. Référence en langue étrangère (Nom du livre ou publication), au complet, transcrire en caractères romains. same as title Reference in English or French - Référence en anglais ou français Publisher - Editeur Page Numbers in original DATE OF PUBLICATION Numéros des pages dans VNIRO DATE DE PUBLICATION l'original 1-173 Year Issue No. Volume Place of Publication Année Numéro Number of typed pages Lieu de publication Nombre de pages USSR dactylographiées 1986 474 Requesting Department Translation Bureau No. D F 0 3287260 Ministère-Client Notre dossier no Branch or Division I P B Translator (Initials) N. -
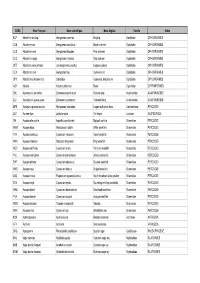
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Hatchlings Grow to Adult Size in 11 to 13 Months, Depending on Environmental Temperature Conditions
click for previous page - 37 - Sepia brevimana Steenstrup, 1875 SEP Sep 12 Sepia brevimana Steenstrup, 1875. Synonymy : Sepia rostrata (pars) Ferussac & Orbigny, 1848; Sepia winckworthi Adam, 1939. FAO Names : En - Shortclub cuttlefish Fr - Seiche petites mains Sp - Sepia mazicorta Diagnostic Features : Mantle broad, its dorsal margin acuminate, strongly projecting anteriorly; posteriorly very pointed due to the long spine. Tentacular club short, with a well developed swimming keel extending proximally beyond base; dorsal protective membrane as broad as sucker-bearing surface; suckers very small, subequal; 6 to 8 suckers in oblique transverse rows. Geographical Distribution : Indo-Malayan region, along the northern coast of the Indian Ocean, from Singapore westwards probably to the west coast of India. Habitat and Biology : A small, demersal species, restricted to shallow, costal waters down to 30 m depth. Spawning extends almost throughout the year; in eastern Indian waters severa1 peaks have been observed between July and February (Silas et al. 1982). Hatchlings grow to adult size in 11 to 13 months, depending on environmental temperature conditions. Off Madras, they attain 2.9 to 3.4 cm within 6 months, 5.6 to 5.8 cm at the end of 12 months and about 7.5 cm after 18 months. Size : Maximum mantle length 10 cm; maximum length in the Indian trawl fishery, 8.5 cm off Madras, and 9.5 cm off Waltair (northeast India). Common size ranges from about 4 to 7 cm. cuttlebone Length at first maturity for males is 5.6 cm off Madras and 6.2 cm off Waltair; for females, 5.9 cm and 6.3 cm, respectively.